Vancomycin – most common
Captopril
Trimethoprim/sulfamethoxazole
Phenytoin
Diclofenac
The most common disease associated with LABD is ulcerative colitis. The relationship is unclear, and abnormal IgA1 production in the gastrointestinal tract is thought to be responsible [12]. LABD has also been reported with lymphoid [13–15] and nonlymphoid [16, 17] malignancies, although a definite association is yet to be established.
A strong association with HLA Cw7, B8, and DR3 has been found by Collier and colleagues. They also found that more CBDC patients possessed HLA B8, DR3, and DQ2 than adult LABD patients, suggesting that these play a role in early disease presentation. Patients with a rare TNF2 allele were associated with longer disease duration [18].
33.5 Clinical Manifestations
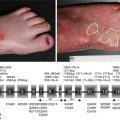
The clinical presentation of LABD is heterogeneous. Tense clear or hemorrhagic vesicles and bullae on normal, erythematous, or urticarial skin are typical (Fig. 33.1), but erythematous macules, papules, plaques (Fig. 33.2), morbilliform [19], and targetoid [20] lesions also occur. The distribution of lesions in LABD bears a resemblance to dermatitis herpetiformis (DH), and in fact LABD was initially thought to be a variant of DH [21]. The distinguishing feature of LABD is the absence of a gluten-sensitive enteropathy.
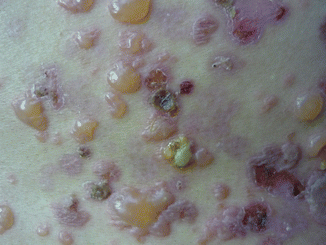
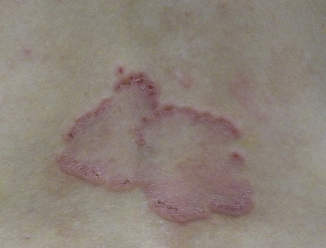

Fig. 33.1
Blisters in different levels of evolution – intact and ruptured blisters, crusted erosions, patches, and dyspigmentation

Fig. 33.2
An annular plaque on the back in linear IgA bullous dermatosis
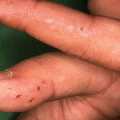
Onset is usually abrupt but may be insidious, with variable prodromal pruritus or a burning sensation. In adults, the most commonly affected areas are the trunk (Fig. 33.1) and extensor extremities, but involvement of the scalp, groin (Fig. 33.3), buttocks, hands, and feet is usual. Blisters, urticarial plaques (Fig. 33.4), and papules arise from normal or within inflammatory areas. The characteristic “string of pearls” sign (Figs. 33.5 and 33.6) representing annular plaques with peripheral vesiculation that is seen commonly in children is less often manifested in adults.
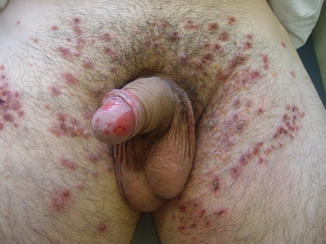
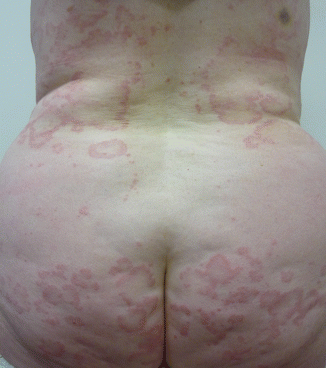
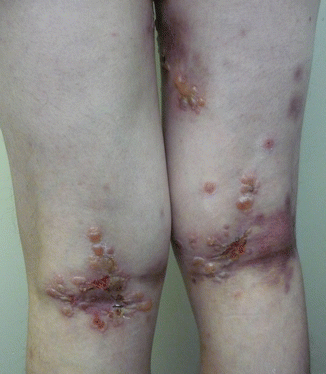
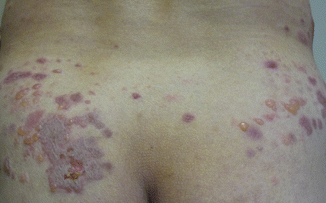

Fig. 33.3
Vesicles and crusted erosions in the pubic and inguinal areas of a man with linear IgA bullous dermatosis

Fig. 33.4
Patient with linear IgA bullous dermatosis with urticarial and annular plaques on the back

Fig. 33.5
The string of pearls sign. New tense blisters emerging from the periphery of previous lesions seen as a collaret of blisters

Fig. 33.6
The string of pearls sign evident on the left buttock of this man with linear immunoglobulin A bullous dermatosis
Mucosal lesions can range from asymptomatic lesions to severe erosions and ulcerations and should be distinguished from mucous membrane pemphigoid (MMP) [22]. They are more common in adults, occurring in up to 80 % [5]. Retrospective studies have found that mucosal lesions in CBDC range from 3 % [23] to 64 % [5]. The oral and ocular mucosae are usually affected; however, any mucosal surface can be affected. Erosions or ulcers are more commonly seen than intact blisters. Oral mucosal lesions usually involve the palate, palatine arches, and the buccal mucosa [7]. Ocular manifestation includes dry eye, foreign body sensation, conjunctival scarring with trichiasis, entropion, corneal opacification, neovascularization, and potential blindness [24]. Oral and ocular mucosal lesions may appear independently or concurrently with cutaneous lesions or may herald the disease.
33.6 Drug-Induced LABD
Mucocutaneous manifestations of drug-induced LABD are comparable to those of the idiopathic form [1, 7]. Idiopathic LABD presents with bullae that tend to be chronic, whereas drug-induced LABD usually appear acutely and resolve with discontinuation of the implicated agent. Over the last 30 years, more than a hundred drug-induced LABD cases have been reported; however, only a few cases have had conclusive evidence of causality. The best-documented drug is vancomycin, where clinical latency after ingestion of the drug ranges from 2 to 21 days, followed by captopril and trimethoprim/sulfamethoxazole [1].
33.7 Diagnosis
Since LABD clinically resembles other mucocutaneous disorders and histopathologic features are nonspecific, confirming a diagnosis of LABD is contingent on immunofluorescence studies. Direct immunofluorescence (DIF) showing linear deposition of IgA along the basement membrane zone is the gold standard for diagnosis [21]. Cases incited by drugs especially vancomycin should be ruled out.
33.8 Pathology
Routine histopathology classically shows subepidermal blistering with a neutrophilic infiltrate. In DH, neutrophils are also observed but are usually much less diffuse and are more common in papillary tips [5, 26]. Eosinophils may occasionally be present and this complicates differentiation from BP.
Perilesional DIF studies should be carried out. The back, if uninvolved, offers a suitable site for biopsy, while the forearm is avoided [27], as antigen expression is least detected in this anatomical site. Pathognomonic homogeneous linear deposits of IgA are seen on DIF, but sometimes IgG, C3, or IgM are detected [5]. A finding of a granular pattern of IgA deposition should alert one to an alternative diagnosis of DH.
Serum and, rarely, blister fluid [28] are used for indirect immunofluorescence (IIF) to detect circulating IgA1 antibodies that target a 97 kDa antigen and a 120 kDa antigen in the basement membrane zone. IIF tends to be more positive in children (≥75 %) than in adults (30 %) [5] and should be done on salt-split human skin (SSS) for increased sensitivity. Majority will show binding to the epidermal side of SSS.
Immunoelectron microscopy shows blister formation in the lamina lucida, beneath the lamina densa [29], and the basal surface of the hemidesmosome [30]. This heterogeneity in the localization of target antigens and autoantibodies may be explained by a recent finding that LAD antigens are expressed by both human keratinocytes and fibroblasts in culture [31]. Immunoblotting may also be done, but this is not commonly used.
33.9 Differential Diagnosis
The closest differentials to LABD are DH and BP. As mentioned the presence of a gluten-sensitive enteropathy favors a diagnosis of DH. Immunofluorescence studies will show a linear deposition in the basement membrane zone of IgG in BP and of IgA in LABD. There is also an IgA variant of epidermolysis bullosa acquisita (EBA).
Bullous pemphigoid, like LABD, is a subepidermal autoimmune blistering disease, but it usually affects people over the age of 80, shows more eosinophils on histology, and will have a linear deposition of predominantly IgG and C3 and not IgA in the basement membrane zone on direct immunofluorescence. A clinical picture compatible with LABD showing IgG autoantibodies on immunofluorescence studies that responds well to sulfones has been termed mixed immunobullous disease [32].
Predominantly mucosal LABD can mimic mucous membrane pemphigoid clinically [22]. Immunofluorescence will distinguish the two.