Lichen Planus: Introduction
|
Lichen planus (Greek leichen, “tree moss”; Latin planus, “flat”) is a unique, common inflammatory disorder that affects the skin, mucous membranes, nails, and hair. The appearance of lichen planus-like lichenoid dermatoses has been likened to the scurfy, finely furrowed, dry excrescences of the symbiotic vegetation known as lichen. Although this morphologic comparison may be antiquated, lichen planus is a distinctive entity with prototypic “lichenoid” papules that show distinctive color and morphology, develop in typical locations, and manifest characteristic patterns of evolution. Microscopic features are also distinctive, although the microscopic pattern of inflammation and skin response is shared by several dermatoses. The term lichenoid reaction1 is the histologic description used to capsulize the pathologic characteristics of skin diseases resembling lichen planus.
Historical Aspects
The exact incidence and prevalence of lichen planus are unknown, but the overall prevalence is believed to be somewhat around 1% of the general population. Estimates between 0.14% and 1.27% have been reported worldwide and approximately 0.44% in the United States. No racial predilection has been observed.2,3
At least two-thirds of cases occur between the ages of 30 and 60 years of age. No sexual predilection is evident. Females are usually affected in their 50s and 60s, whereas males develop lichen planus at a somewhat earlier age. The disease is less common in the very young and the elderly. The development of lichen planus may be affected by seasonal or environmental factors.2
Fewer than 100 cases of familial lichen planus have been reported. The familial form tends to be more protracted and severe and presents in erosive, linear, or ulcerative patterns or with atypical features affecting young adults and children.4 Some believe that the familial form represents a separate, unique dermatosis. Different HLA haplotypes were reported in familial lichen planus, including HLA-B7, -Aw19, -B18, and -Cw8. In nonfamilial lichen planus, HLA-A3, -A5, -A28, -B8, -B16, and -Bw35 are more common.5 HLA-B8 is more common in patients with oral lichen planus as a sole manifestation, and HLA-Bw35 is more strongly associated with cutaneous lichen planus.
Etiology and Pathogenesis
It is evident that specific immunologic mechanisms control the development of lichen planus. T-cell mediated pathologic alterations involving proinflammatory and counterregulatory mechanisms function in the pathogenesis of lichen planus.6 No consistent alterations in immunoglobulins (Igs) have been shown in lichen planus, and humoral immunity most likely is a secondary response in immunopathogenesis.
Cell-mediated immunity, on the other hand, plays the major role in triggering the clinical expression of the disease. Both CD4+ and CD8+ T cells are found in lesional skin of lichen planus. Progression of disease may lead to preferential accumulation of CD8+ cells. The majority of lymphocytes in the infiltrate of lichen planus are CD8+ and CD45RO (memory) positive cells and express the α–β T-cell receptor (TCR), and in a minority, the γ–δ receptor. This later cell subtype is not normally found in healthy skin. These cells are considered responsible for the development of the most characteristic change observed in the lichenoid reaction, namely, apoptosis.7 The inflammatory process that leads to apoptosis is complex and not fully understood. The epithelium–lymphocyte interaction can be divided into three major stages: (1) antigen recognition, (2) lymphocyte activation, and (3) keratinocyte apoptosis.
It is evident that the majority of T cells in the infiltrate of lichen planus, both within the epithelium and adjacent to damaged basal keratinocytes, are activated CD8+ cytotoxic lymphocytes. Evidence from oral lichen planus suggests that CD8+ lesional T cells recognize a lichen planus-specific antigen associated with major histocompatibility complex (MHC) class I on lesional keratinocytes. The nature of this antigen is unknown. Theoretically, the antigen may be an autoreactive peptide, thus classifying lichen planus as an autoimmune disease. Alternatively, it may represent an exogenous antigen such as an altered protein, drug, contact allergen, viral or infectious agent, or an unidentified immunogenic target. Innate immune responses that become activated by exogenous stimuli in a genetically susceptible individual may trigger the development of lichen planus and, further, involves a small but significant lesional population of CD56+CD16− NK lymphocytes that secrete interferon-γ, tumor necrosis factor (TNF)-α, and only minimal amounts of IL-22, IL-17, or IL-4.
The role of T-helper (CD4) cells in the pathogenesis of lichen planus is not fully defined. T cells may become activated via professional antigen-presenting cells such as Langerhans cells, dendritic cells, or accessory cells such as epidermal keratinocytes in association with members of the MHC II and specific cytokines. T-helper lymphocytes may also propagate CD8+ cytotoxic lymphocytes through cellular cooperation and release of cytokines.
The nature of antigenic stimulation is not known. Contact sensitizers such as metals could act as haptens and elicit an immunologic response. Enhanced lymphocyte reactivity to inorganic mercury, a component of dental amalgam, has been found in patients with oral lichenoid reactions. Low-grade chronic exposure to mercury, and possibly to other metals such as gold, may stimulate a lymphocytic reaction that manifests as lichen planus. A list of contact chemicals and drugs that can elicit lichenoid reactions is discussed in Section “Drug-Induced Lichen Planus.” With more widespread use of biologics for various chronic inflammatory diseases, cases of lichenoid, interface dermatitis are being recognized and implicate dysregulated cytokine production, including upregulation of type-I interferon expression in the setting of tumor necrosis factor (TNF)-α blockage.8 The role of infection in the development of lichen planus has been repeatedly raised over the years. Though provocative, no conclusive evidence has molecularly linked lichen planus to any of the following infections or microorganisms: syphilis, herpes simplex virus 2, human immunodeficiency virus (HIV), amebiasis, chronic bladder infections, hepatitis C virus (HCV), Helicobacter pylori, or human papillomavirus.
Following antigen recognition, CD8+ T cells are activated. Activated cytotoxic lymphocytes undergo lesional tissue clonal expansion, leading to oligoclonal and occasionally to monoclonal proliferation as detected by analysis of the TCR-γ chain gene products.
Activated lymphocytes, both by helper subsets (Th1 and Th2) and cytotoxic-suppressor cells, release soluble mediators (cytokines and chemokines), such as interleukin (IL)-2, IL-4, IL-10, interferon (IFN)-γ, tumor necrosis factor (TNF)-α, and transforming growth factor-β1, that attract lymphocytes and regulate their biologic activities within and adjacent to the epithelium. Both pro- and anti-inflammatory cytokines, i.e., mixed Th1 and Th2 cytokine products, are generated simultaneously. The balance between lymphocytic activation and down regulation determines the clinical behavior of the disease. IFN-γ, produced by T-helper cells during the antigen recognition stage, induces keratinocytes to produce lymphotoxin-α and TNF-α, and to upregulate MHC class II, thus increasing interactions with T-helper cells. Furthermore, IFN-γ upregulates the expression of intercellular adhesion molecule-1 and vascular cell adhesion molecule-1 by basal keratinocytes, Langerhans cells, and other macrophage-dendritic cells. IFN-γ and IL-4 are elevated in saliva from oral lichen planus. Intercellular adhesion molecule-1 is a ligand for the β2-integrin, leukocyte function-associated antigen-1, on the surface of lymphocytes, which further enhances the interaction of these lymphocytes with the antigen-presenting cells. Laminin-5 and collagen types IV and VII are increased in lesional lichen planus and serve as ligands for β1-integrin on the surface of lymphocytes, thus allowing for enhanced association of lymphocytes with the basement membrane. Integrin α3 is present on activated, skin-homing T cells and may localize these effector cells to the epidermal–dermal interface and basement membrane, which contain epiligrin/laminin-5, a ligand for this integrin. This close interaction between lymphocytes and basement membrane targets metalloproteinases produced by lymphocytes to alter extracellular matrix proteins and integrins, and the process eventuates in apoptosis, basement membrane disruption, reduplication, and subepidermal cleft formation (see Section “Pathology”). TNF-α upregulates the expression of matrix metalloproteinase (MMP)-9 mRNA in lesional T lymphocytes, thus further enhancing basement membrane disruption (see Section “Keratinocyte Apoptosis”).9
Keratinocytes also participate in the response by producing IL-1β, IL-4, IL-6, granulocyte–macrophage colony-stimulating factor, and TNF-α. These cytokines further activate tissue macrophages and peripheral blood mononuclear cells and upregulate expression of cell surface adhesion molecules and migration activity. Keratinocyte-produced cytokines also upregulate expression of specific keratin genes. Keratin (K)17, usually restricted to adnexal structures, is variably expressed in the basal and suprabasal layers of the interfollicular epithelium of affected epidermis. K6 and K16 also become detectable in the basal and suprabasal layers. K4 and K13 are reduced in the suprabasal compartment in areas with orthokeratosis, associated with increased production of K1 and K10.
The exact mechanisms used by activated cytotoxic T cells to trigger apoptosis of keratinocytes are not completely known. Possible mechanisms include: (1) T-cell secreted TNF-α binding to the TNF-α R1 receptor on the keratinocyte surface, (2) T-cell surface CD95L (Fas ligand) binding CD95 (Fas) on the keratinocyte, and (3) T-cell secreted granzyme B entering the keratinocyte via perforin-induced membrane pores. In lichen planus, granzyme B predominates in lesional epidermis versus perforin in graft-versus-host disease.10 All these mechanisms may activate the keratinocyte caspase cascade, resulting in keratinocyte apoptosis. Caspase 3 is frequently found to be elevated in both cutaneous and oral lichen planus lesional skin.11
Recruited lymphocytes may further contribute to apoptosis via a different mechanism, the loss of a basement membrane-derived cell survival signal that normally prevents the onset of apoptosis. Hence, basement membrane disruption may trigger apoptosis. Principle mediators are the MMPs, a family of zinc-containing endoproteinases that primarily function to degrade connective tissue matrix proteins. The action of these enzymes is also regulated by inhibitors such as tissue inhibitors of metalloproteinases (TIMPs) (see Chapter 63). It has been shown that lesional T cells in lichen planus have higher MMP-9 levels than peripheral blood T cells. Lesional T-cell MMP-9 activity increases following stimulation with TNF-α, but not TIMP-1, an inhibitor for MMP-9. These observations suggest that T-cell secreted MMP-9 disrupts epithelial basement membranes, blocking cell survival signals to keratinocytes and inducing apoptosis.
Various other environmental, behavioral, or infectious factors have been observed on occasion to be associated with the development or exacerbation of lichen planus. However, no well-established association has been documented between emotional stress, tobacco use, or oral or gastrointestinal candidiasis and development of lichen planus.
Recent studies using microarray technologies to examine and characterize in greater detail various inflammatory and immune-mediated skin diseases have provided considerable new insights into mechanisms mediating-specific inflammatory responses, including lichen planus, oral lichen planus, lichen planopilaris as well as lichenoid eruptions, and interface dermatitis. Lichen planus was distinguished from atopic dermatitis, psoriasis, and healthy skin by elevated expression of type I interferon-induced genes and a specific cytokine expression pattern.12 CXCL9, the ligand for CXCR3 was the most significant marker for lichen planus. In the scarring alopecias, specifically lichen planopilaris, microarray analysis implicates the loss of peroxisome proliferator-activated receptor (PPAR) γ expression in contributing to proinflammatory lipid accumulation and inflammatory cell infiltration leading to pilosebaceous destruction.13 Recently, Brn2, a transcription factor expressed by keratinocytes as well as lymphocytes, when overexpressed in epidermis, has been found to not only cause epidermal changes characteristic of lichen planus but also inflammatory, interface cellular reactivity.14
Clinical Findings
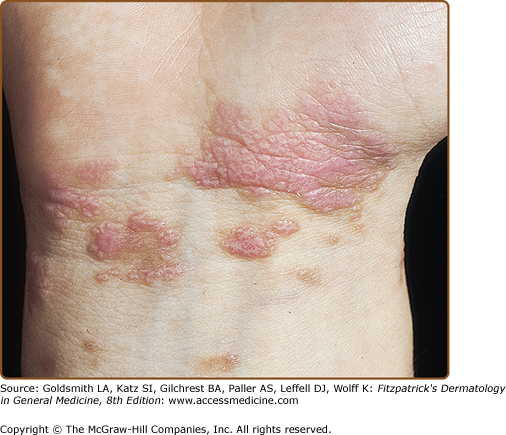
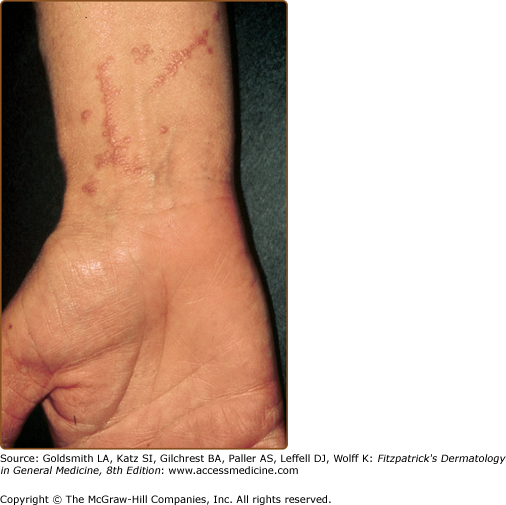
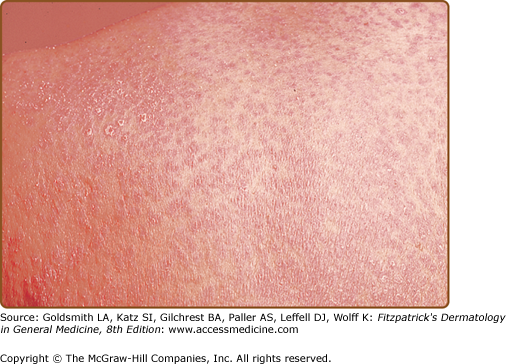
The classic cutaneous lesion of lichen planus is a faintly erythematous to violaceous, flat-topped, polygonal papule, sometimes showing a small central umbilication (Fig. 26-1). Papules are grouped and tend to coalesce. A thin, transparent, and adherent scale may be discerned atop the lesion. Fine, whitish puncta or reticulated networks referred to as Wick-ham striae are present over the surface of many well-developed papules (see Fig. 26-1). These are considered to be highly characteristic and are more easily observed after applying oil, xylene, or water and visualizing the lesions with a magnifying lens or a handheld dermatoscope. The surface alteration may result from localized thickening of the keratohyalin-containing cell layers of the stratum granulosum, although a focal increase in the activity of lichen planus may account for the morphologic alteration of Wickham striae.
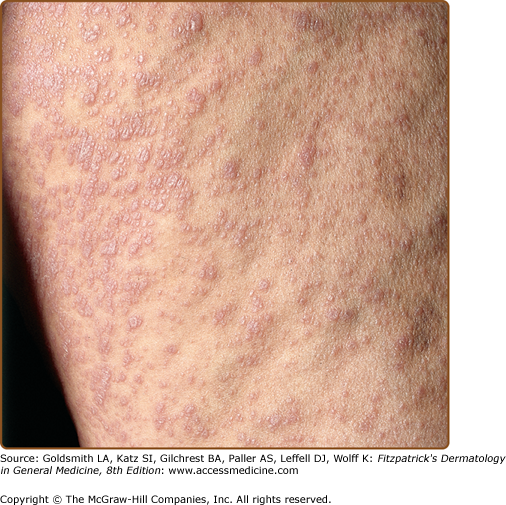
Lichen planus usually develops over several weeks. Sometimes multiple lesions develop rapidly with dissemination following the initial appearance. In generalized disease (Fig. 26-2), the eruption often spreads within 1–4 months from onset. The initial lesions almost always appear on the extremities, especially the legs.
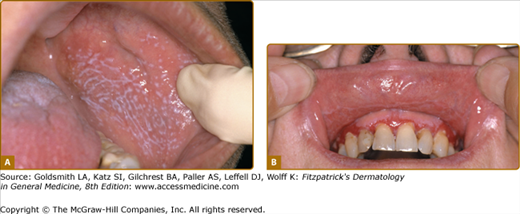
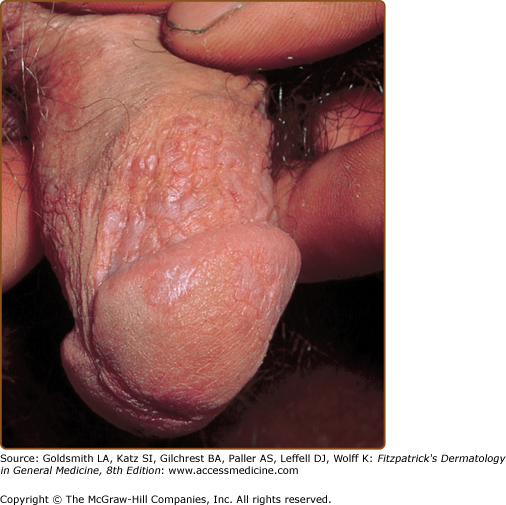
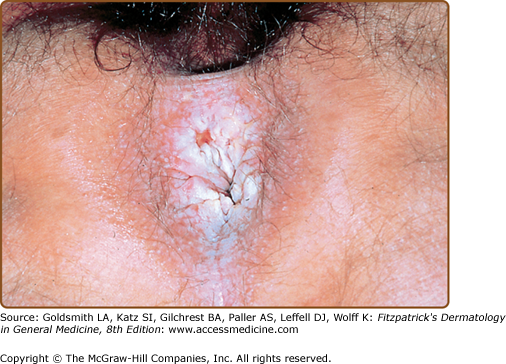
The lesions are usually distributed symmetrically and bilaterally over the extremities. Lichen planus tends to involve the flexural areas of the wrists, arms, and legs (see Fig. 26-1). The thighs, lower back, trunk, and neck may also be affected. Oral mucous membranes (Fig. 26-3) and the genitalia (Fig. 26-4) are additional sites of involvement. The face is usually spared in classical cases, and palmoplantar involvement is unusual. Inverse lichen planus usually affects the axillae, groin, and inframammary areas.
Lichen planus tends to be quite pruritic, although some patients are completely asymptomatic. The degree of pruritus is generally related to the extent of involvement, with more intense pruritus in generalized form. An exception is hypertrophic lichen planus, which is more localized but extremely pruritic. Oral involvement is generally asymptomatic unless erosions or ulcers developed, after which it becomes extremely painful. In the acute, evolving stages of the disease, scratching, injury, or trauma may induce an isomorphic (Koebner) response (Fig. 26-5). Lichen planus usually heals with hyperpigmentation (see Fig. 26-1), which is more prominent among patients with darker skin color. Hypopigmentation uncommonly develops after resolution of lesions.
Most reports of lichen planus in children have come from the Indian subcontinent, suggesting that children of South Asian origin are more susceptible to developing lichen planus.15 Although the clinical and pathologic features of childhood lichen planus are similar to those in adults, scalp, nail, and hair involvement are not common. Mucous membrane involvement, thought to be rare, may occur in up to one-third of patients. The hypertrophic variant occurs in one-fourth of children with lichen planus.
Many variations in the clinical presentation have been described and are generally categorized according to (1) the configuration of lesions, (2) the morphologic appearance, or (3) the site of involvement. These variations are patterned by subtle or unknown properties of the disease. The prototypic papule can be altered or modified in configuration, morphology, or anatomic distribution.
Annular lesions occur in approximately 10% of cases and commonly develop as arcuate groupings of individual papules that develop rings or peripheral extension of clustered papules with central clearing. They tend to occur in blacks and are more common on the penis and scrotum (see Chapter 75). It occurs also when larger lesions on trunk and extremities reach 2–3 cm in diameter and become hyperpigmented with a raised outer rim. Actinic lichen planus (see below), seen in subtropical zones on sun-exposed, dark-skinned young adults and children, is frequently annular in shape.
Papules of lichen planus may develop a linear pattern secondary to trauma (koebnerization) (see Fig. 26-5) or, rarely, in less than 0.2% of cases as a spontaneous, isolated eruption, usually on the extremities following Blaschko lines. The segmental formation is thought to be due to a postzygotic mutation that affects one of the genes predisposing its development that lead to the formation of a keratinocyte clone that is more susceptible to development of lichen planus.16 Drug-induced linear lichen planus has also been reported.17,18
It is important to differentiate linear lichen planus from nevus unius lateris, lichen striatus, inflammatory linear verrucous epidermal nevus, linear psoriasis, and linear Darier–White disease, which have different presentations clinically and histopathologically.
Hypertrophic lichen planus (lichen planus verrucosus) usually occurs on the extremities, especially the shins and interphalangeal joints, and tends to be the most pruritic variant (Fig. 26-6).19 Lesions are thickened and elevated, purplish or reddish-brown in color, and hyperkeratotic. Occasionally, verrucous plaques develop. Lesions may show accentuated and elevated follicular induration and chalk-like scale. This variant usually heals with scar formation and hyper- or hypopigmentation. Chronic venous insufficiency is frequently present.
The atrophic variant is rare and is characterized by the presence of a few well-demarcated, white-bluish papules or plaques, sometimes more erythematous, with central superficial atrophy.2 The lesions are a few millimeters wide but may coalesce to form larger plaques. They are most common on the lower extremities or trunk. The lesions often resemble lichen sclerosus et atrophicus. However, the histologic features are diagnostic.
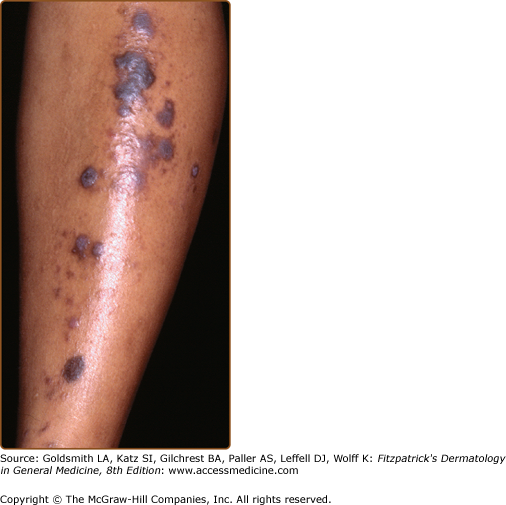
The development of vesicles and bullae in lichen planus is rare. (Fig. 26-7). The bullae, which appear most commonly on the extremities, arise from existing papules of lichen planus and rarely from normal-appearing skin. They may appear suddenly during an acute flare of disease and are usually associated with mild constitutional symptoms. These lesions usually resolve in a few months. Bulla arising in oral lichen planus can lead to painful erosions. Histologically, subepidermal separation is present in addition to typical features. Bullae formation does not necessarily indicate a longer duration of the disease. Bullae arising from normal skin are more characteristic of lichen planus pemphigoides and should be differentiated by direct and indirect immunofluorescence (see below).
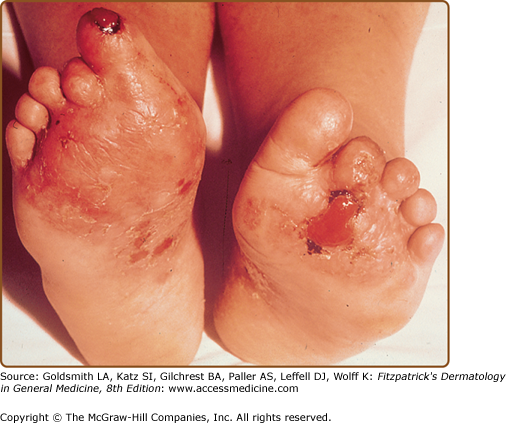
A rare variant presents with chronic, painful bullae and ulcerations of the feet with often, cicatricial sequelae (Fig. 26-8). Patients typically have nails, and mucosal involvement in addition to classical skin lesions, which often aids in establishing the diagnosis. Permanent loss of toenails and cicatricial alopecia of the scalp are common. Squamous cell carcinoma (SCC) may develop in lesions of ulcerative lichen planus.20
Erosions and ulcerations may also develop in more severe cases of oral lichen planus.
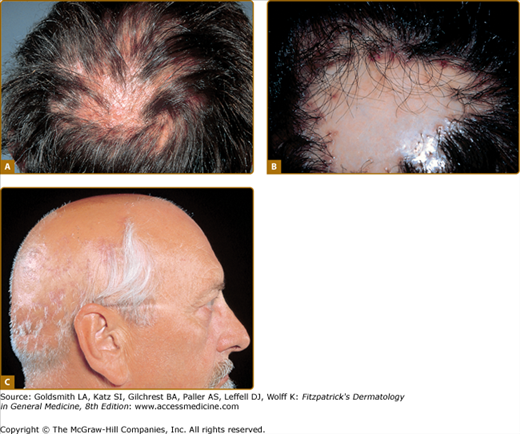
Follicular lichen planus may occur alone or in association with other forms of cutaneous or mucosal lichen planus.16 The term lichen planopilaris has been used to describe this distinctive variant, but other terms include lichen planus follicularis, peripilaris, and acuminatus. Individual keratotic follicular papules and studded plaques are seen. Sites of predilection include the trunk and medial aspects of the proximal extremities.21 Follicular lichen planus may affect the scalp with the development of cicatricial alopecia (see Section “Lichen Planus of the Scalp”; Fig. 26-9). The triad of follicular lichen planus of skin (lichen planus spinulosus) and/or scalp, multifocal cicatricial alopecia of the scalp, and nonscarring alopecia of the axillary and pubic areas, has been described as Graham–Little–Piccardi–Lassueur (or Graham–Little–Feldman) syndrome. Sometimes more typical lesions of the skin and nails are seen. Other variants of follicular lichen planus include the lichen planus follicularis tumidus with oval pseudotumoral plaques of the mastoid area, postmenopausal frontal fibrosing alopecia,22 and lichen planoporitis, with the lichenoid reaction centered over the acrosyringium and eccrine ducts entering the epidermis.
Figure 26-9
Lichen planus. A. Irregular patches of alopecia with violaceous papules and coalescing plaque of lichen planus within scalp. B. Lichen planopilaris resulting in atrophic, scarred, porcelain-colored area centrally with bordering follicular involvement consisting of dusky erythema, perifollicular hyperkeratosis and scale, and follicular convergence resulting in doll’s hair formation. C. Extensive hair loss leading to pseudopelade of Brocq appearance. Several remaining tufts of hair showing violaceous erythema and disease activity.
This variant is characterized by hyperpigmented, dark-brown macules in sun-exposed areas and flexural folds. This entity tends to occur in patients with darker pigmented skin. Histologically, an atrophic epidermis, a vacuolar alteration of the basal cell layer with a scarce lymphohistiocytic lichenoid infiltrate, and pigment incontinence are seen. This variant bears significant similarity to ashy dermatosis or erythema dyschromicum perstans. It may represent an overlap in the phenotypic spectrum of lichenoid inflammation in darkly pigmented skin, with ethnic and genetic factors influencing the expression of disease (see Chapter 75).23 In cases where the inflammatory phase was minimal, such as lichen planus “invisible de Gougerot,” the pigmentation may be the only sign of the disease.
Also known as lichen planus subtropicus, lichen planus tropicus, summertime actinic lichenoid eruption, lichen planus actinicus, lichen planus atrophicus annularis, and lichenoid melanodermatosis. Actinic lichen planus affects young people of the Middle East in spring and summer, where sunlight appears to have a precipitating effect. Exposed areas of the face, dorsal hands and arms, and the nape of the neck are usually affected. Papules are hyperpigmented with violaceous-brown color and a thready, rolled edge showing well-defined borders. Annular lesions are common, but pigmented and linear forms were seen.24,25 Typical lichen planus lesions may be present over the extremities. Pruritus and scaling are minimal.
A perforating variant has been described in which transepidermal elimination of lichen planus-like inflammatory tissue is observed. Guttate lichen planus is another variant that resembles guttate psoriasis but with the characteristic lichenoid histology. Exfoliative and exanthematous forms are very rare and may represent manifestations of lichenoid drug reactions (Fig. 26-10). The rare entity of invisible lichen planus describes lesions that are not perceptible with visible light illumination but become apparent with Wood’s lamp examination. Pruritus is present and biopsy evaluation shows lichenoid histology. This entity may be a minimal variant of lichen planus “invisible de Gougerot.”
Lichen planopilaris (LPP) or follicular lichen planus may affect the scalp in a distinctive clinical and histologic pattern that affect women more than men.26–30 LPP can be subdivided into three variants: (1) classic LPP, (2) frontal fibrosing alopecia, and (3) Lassueur–Graham–Little–Piccardi syndrome. In classic LPP, individual keratotic follicular papules that coalesce and merge over the scalp to form patches are typically seen. Perifollicular erythema and acuminate keratotic plugs are characteristic features. Follicular-centered lesions are usually observed under close inspection of the scalp and orifices, particularly at the margins of the alopecic area or within patches still bearing hair. Cutaneous, nail, or mucous membrane involvement with lichen planus may also be present. Patients present with uni- or multifocal hair loss that may be extensive and sometimes involve the entire scalp (see Fig. 26-9). The condition can have major psychological impact on affected individuals.
End-stage disease is characterized by a nondescript scarring alopecia that has led to the use of several clinical terms describing the entity: lichen planopilaris, folliculitis decalvans et atrophicus, lichen spinulosus at folliculitis decalvans, and Graham–Little syndrome.
Frontal fibrosing alopecia is an uncommon condition characterized by progressive frontotemporal recession due to inflammatory destruction of hair follicles. It is more common in postmenopausal women, but it can occur in younger women. It may be associated with mucocutaneous lichen planus. Recession of the hairline may progress inexorably over many years, but this is not inevitable.22,31
Pseudopelade of Brocq is a rare clinical syndrome of scarring alopecia and fibrosis, in which distinct pathologic features are absent. It is generally accepted that pseudopelade of Brocq is the end stage of follicular fibrosis caused by a primary inflammatory dermatosis such as lichen planus, lupus erythematosus, pustular-scarring forms of folliculitis, or fungal infections, including favus, scleroderma, and sarcoidosis.
Lichen planus can affect the mucosal surfaces of mouth, vagina, esophagus, conjunctiva, urethra, anus, nose, and larynx. Its prevalence is estimated at approximately 1% of the adult population. Oral involvement occurs in approximately 60%–70% of patients with lichen planus. It may be the only manifestation in 20%–30% of patients.
In oral lichen planus, different types have been described, including reticular, plaque-like, atrophic, papular, erosive/ulcerative, and bullous forms (see Fig. 26-3).32,33 The reticular pattern is considered the most common, but patients with erosive form are more likely to seek medical help due to the symptomatology (pain and burning sensation) and chronicity. Most patients have more than one type. The buccal, gingival, and glossal mucosae are the most commonly affected areas. The palate, floor of the mouth, retromolar pads, and lips may also be affected. Gingival involvement may take the form of gingival stomatitis or desquamative gingivitis, and could be the sole presentation in 8% of oral lichen planus. On the other hand, lichen planus is the most common cause for desquamative gingivitis.34
Oral lichenoid reaction is similar clinically and histologically to oral lichen planus, however, with identifiable etiology. Differentiating these two entities is often difficult. It is usually seen on the buccal mucosa adjacent to amalgam dental fillings.35,36 Patch tests frequently show positive reactions to mercury, gold, and other metals.37,38
Bilateral reticular keratotic or atrophic changes of the buccal mucosa and lichenoid atrophic patches over the dorsal tongue have been described in patients with HIV infection. The eruptions usually follow zidovudine or ketoconazole intake.
Esophageal Lichen planus is rare and affects the proximal esophagus. It manifests as progressive dysphagia and odynophagia. Endoscopic findings can include lacy white papules, pinpoint erosions, desquamation, pseudomembranes, and stenosis. Histologically, it shows parakeratosis, epithelial atrophy, and lack of hypergranulosis. Squamous cell carcinoma of esophagus may develop after longstanding disease.39,40
Male genitalia are involved in 25% of cases, and the glans penis is most commonly affected, with annular lesions frequently present (see Fig. 26-4). Anal lesions of mucosal lichen planus present with leukokeratosis, hyperkeratosis, fissuring, and erosions (Fig. 26-11).
Vulvar and vaginal lichen planus is present in over half of patients with oral lichen planus.41 Clinically, the condition is often asymptomatic unless erosions develop. Burning, itching, pain and abnormal discharge then become common.42 Examination reveal patches of leukoplakia or erythroplakia, sometimes with erosions, and, occasionally, as a more generalized desquamative vaginitis. Vaginal adhesions and labial agglutination may result.
The vulvo-vaginal gingival syndrome is characterized by involvement of vulvar and gingival tissues. Erythema and erosions of the gingivae and tongue and, occasionally, white reticulated plaques, is associated with desquamation and erosions of vulva and vagina.43
Conjunctival lichen planus may manifest as cicatricial conjunctivitis. Histologically, irregular thickening with reduplication of the basement membrane is seen. Direct immunofluorescence distinguishes ophthalmic lichen planus from cicatricial pemphigoid.
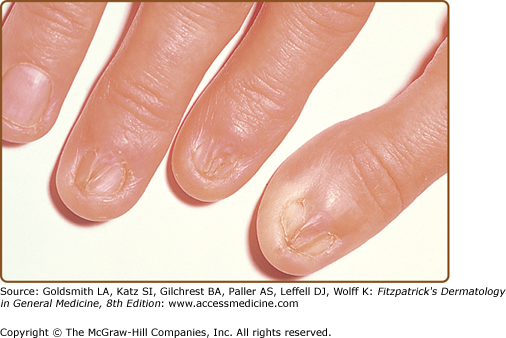
(See also Chapter 89.) Nail involvement occurs in 10%–15% of patients.44 Lichen planus limited to the nails is uncommon, and the initial involvement is followed, in many cases, by the development of typical lesions elsewhere. Lichen planus of nails is infrequent in children. Thinning, longitudinal ridging, and distal splitting of the nail plate (onychoschizia) are the most common findings. Onycholysis, longitudinal striation (onychorrhexis), subungual hyperkeratosis, or even absence (anonychia) of the nail plate can also be seen. Twenty-nail dystrophy (trachyonychia) may represent an isolated nail finding of lichen planus. Psoriasis and alopecia areata can also lead to these distinctive nail changes. Nail loss may result from ulcerative lichen planus involving the nail unit. Pterygium or forward growth of the eponychium with adherence to the proximal nail plate is a classic finding of lichen planus of the nail (Fig. 26-12). An atrophic cicatrizing form of lichen planus with random and progressive nail loss in Asians and blacks has also been reported. The tenting or pup-tent sign is observed as a result of nail bed involvement that elevates the nail plate and may cause longitudinal splitting.2
The inverse pattern occurs only rarely and is characterized by red-brownish, discrete papules, and nodules. The eruption occurs mainly in the flexural areas such as axillae, inframammary, groin, and, less likely, the popliteal and antecubital areas. Koebnerized lesions are occasionally present.
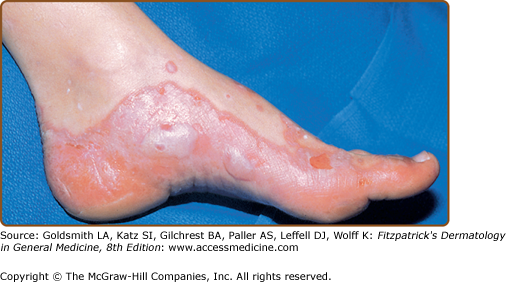
This is a rare variant that is difficult to diagnose if present as an isolated finding. Very pruriginous, erythematous, scaly plaques with or without hyperkeratosis are characteristic. Lesions are often seen on the internal plantar arch. Yellowish, compact keratotic papules or papulonodules are seen on the lateral margins of the fingers and hand surfaces; however, they are less likely to affect the fingertips. They appear like callosities with an inflammatory, erythematous halo. The lesions resemble psoriasis vulgaris, warts, calluses, porokeratosis, hyperkeratotic eczema, tinea, or secondary syphilis.45 Palmoplantar lichen planus may also present as erosive–ulcerative type (see Section “Erosive and Ulcerative Lichen Planus”).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree