Latissimus Dorsi Musculocutaneous Flap Breast Reconstruction
Dennis C. Hammond
The goals of present-day breast reconstruction are more demanding than in previous years when the simple creation of an often shapeless breast mound was all that was possible. Today, advances in technique have allowed various contours and landmarks such as base diameter, inframammary fold location, projection, and a ptotic breast shape to be created, which has resulted in a tremendous improvement in the aesthetic results of breast reconstruction. Central to the realization of these improved results was the description and use of the latissimus dorsi musculocutaneous flap (LDMF).
The LDMF was originally described by Tansini in 1906 for use as an axial musculocutaneous flap to cover mastectomy defects (1). This technique of mastectomy wound management became popular in Europe for a time but eventually fell from favor. The importance of Tansini’s discovery was lost until almost 70 years later, when Olivari redescribed the flap in 1976 (2). In 1977, Schneider et al. (3) described the anatomy of the flap and outlined its use for reconstruction of the breast. Many reports quickly followed documenting the utility of the flap in breast reconstruction (4,5,6). In addition, the first experiences using the LDMF as a free flap were described (7,8,9). Despite this early enthusiasm, use of the LDMF declined after description of the transverse rectus abdominis musculocutaneous (TRAM) flap by Maxwell et al. in 1979 (10). The ability to reconstruct the breast completely with autogenous tissue via the TRAM flap obviated the complications associated with the use of prosthetic devices, which were required when the LDMF was used. As a result, use of the LDMF declined, and the technique was relegated to those few cases that could not be reconstructed with a tissue expander alone or in women who for various reasons were not candidates for TRAM flap breast reconstruction.
More recently, with improvements in tissue expander and implant design, the LDMF has regained popularity and is providing results that are of the same quality as those obtained in TRAM flap breast reconstruction, without the morbidity associated with harvest of the rectus abdominis muscles. In addition, a completely autogenous reconstruction using the LDMF can be performed in selected patients without the use of an implant. As a result, the LDMF has again assumed a prominent role in the armamentarium of the reconstructive breast surgeon.
Anatomy
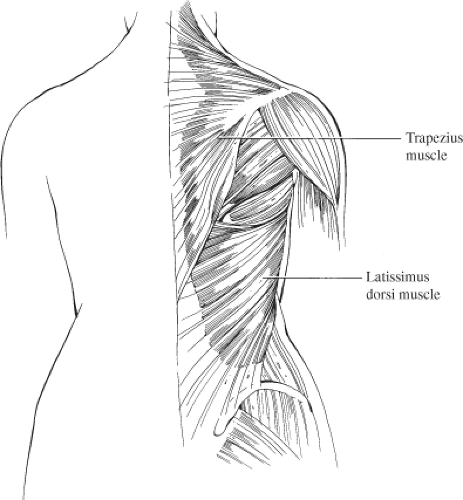
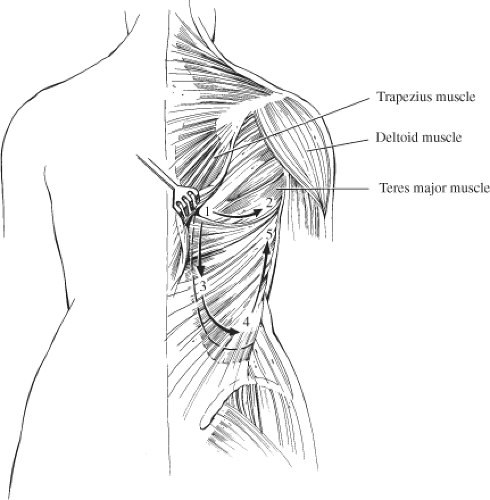
The latissimus dorsi muscle is the largest and most superficial of the muscles of the posterior chest wall (Fig. 44.1). It is a broad, flat muscle that extends from a wide area of origin over the posterolateral thorax. Superiorly, it overlies the tip of the scapula. As it passes toward the axilla, it joins with fibers of the teres major to form the posterior axillary fold. The superomedial corner of the muscle is overlain by the trapezius muscle. Medially, the muscle thins into a fascial aponeurosis that joins with the lumbosacral fascia along the back, which then extends inferolaterally along the iliac crest. Inferolaterally, the muscle thickens and fuses with fibers of the external oblique and intercostal muscles of the lower three to four ribs. The lateral margin of the muscle then extends freely up into the axilla, forming the posterior border of the axilla.
The latissimus overlies the serratus anterior and a portion of the external oblique muscles. At the level of the tenth to the eleventh rib, there is a firm, thick aponeurotic attachment between the serratus anterior and the latissimus, which divides the sublatissimus plane into an inferior and superior portion. This aponeurosis corresponds to the lower border of the serratus anterior. Failure to properly divide this aponeurosis results in inadvertent elevation of the serratus anterior muscle during flap elevation. From this broad origin, the muscle fibers spiral 180 degrees to insert into the intertubercular groove of the humerus via a flat, distinct tendon.
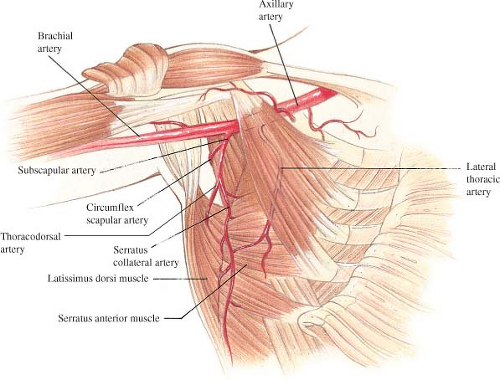
The blood supply to the latissimus dorsi muscle is constant and exhibits no significant anatomic variation that prevents muscle transposition (Fig. 44.2). The subscapular artery arises from the axillary artery to give two main branches: the circumflex scapular and thoracodorsal arteries. The thoracodorsal artery and vein then give off a branch to the serratus muscle shortly before entering the underside of the latissimus muscle 9 to 11 cm below the axillary artery. In cases in which the thoracodorsal pedicle has been divided, reversal of flow through this serratus branch provides adequate blood flow to the flap, allowing it to be safely transposed anteriorly (11). Once in the muscle, the vascular pedicle bifurcates into a transverse and lateral branch, which then arborizes within the muscle to produce extensive intramuscular collateralization (12,13,14). A secondary blood supply enters the underside of the muscle via paraspinous perforators off the posterior intercostal arteries located 4 to 5 cm from the midline of the back. These perforators allow the flap to be elevated as a foldover flap to cover midline back defects (15).
Numerous musculocutaneous perforators extend from this rich vascular plexus into the overlying tissues, forming anastomoses with the circumflex scapular artery within the dorsal thoracic fascia (16,17). This allows skin islands to be safely designed anywhere within the margins of the muscle, although the most reliable location is over the lateral aspect of the muscle corresponding to the course of the lateral branch of the thoracodorsal artery (12,13,14).
The latissimus dorsi muscle is an adductor and medial rotator of the humerus. It also assists in securing the tip of the scapula against the posterior chest wall. Transposition of this muscle anteriorly has been shown to be well tolerated by patients and results in only a minimal functional deficit (18,19), although dynamic weakness in shoulder extension and adduction may occur (20).
Indications
The indications for use of the LDMF in breast reconstruction vary and depend in part on the preferences of both the reconstructive surgeon and the patient. The flap has a vigorous and reliable blood supply, which allows it to be used safely even in heavy smokers. In these patients, use of the LDMF with a shaped, textured expander or prosthesis may be a better option than the pedicled TRAM flap procedure due to the increased incidence of complications noted in smokers with the TRAM flap. The LDMF has also proven to be useful in patients who might otherwise be candidates for tissue expander reconstruction alone due to the additional soft tissue provided by the flap, which can improve the aesthetic quality of the reconstruction over that obtained with the addition of only the prosthesis (21). The LDMF is an excellent option in patients who have experienced partial TRAM flap necrosis, which, after debridement, leaves a distorted reconstructed breast. Filling in the tissue defect with an autogenous LDMF can salvage the reconstruction and provide excellent results. Likewise, using the LDMF to fill in a lumpectomy defect can provide excellent results, even with a history of previous radiation. The LDMF can also be used to reconstruct patients who have already undergone a TRAM flap procedure or who are otherwise not candidates for a TRAM flap due to illness, insufficient lower abdominal tissue, or patient preference.
Operative Technique
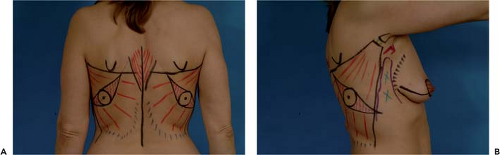
Elevation and anterior transposition of the LDMF is straightforward and can be accomplished easily after surrounding landmarks have been identified. Accurate preoperative markings are vital to properly position the skin island and should always be made with the patient upright. The superior margin of the flap is identified by locating the tip of the scapula and drawing a curved line across this landmark up into the axilla over the top of the posterior axillary fold. The lateral margin is identified by drawing a straight line along the anterior margin of the posterior border of the axilla down to the iliac crest. Between these lines, as well as the posterior border of the iliac crest, and the midline of the back lies the latissimus dorsi muscle. The skin island can be oriented in essentially any direction. One strategy involves placing the long axis of the ellipse horizontally across the back such that the donor site scar hides in the bra line. Unfortunately, this often results in bunching of the skin closure at the anterior end of the scar under the arm. This irregular folding of skin can detract from the overall result. A better option is to respect the relaxed skin tension lines as they extend across the back. These lines can be identified by gently taking traction off the skin of the back by pinching together the subcutaneous layer with the index finger and thumb. The lines can then be seen easily. Orienting the long axis of the ellipse along this line results in a fine line scar with no bunching or “dog ear” formation at either end. The scar ends up running obliquely across the back from superomedial to inferolateral but is usually of sufficient quality that it is imperceptible. The width of the ellipse will vary from patient to patient, depending on the body habitus and the degree of elasticity of the skin. In cases of delayed reconstruction, the width of the ellipse is taken as wide as possible to still allow primary closure of the back donor site. This can be assessed by simply pinching the skin of the back together along the long axis of the ellipse to gauge how much can safely be removed. In immediate reconstruction, usually all that is required is a circular-shaped skin island designed to fill in the defect created by removing the nipple-areola complex (NAC). In these cases, the ellipse does not have to be as wide, and the skin island is designed with sufficient width to allow easy closure of the back donor site. Last, the anterior border of the muscle is marked and the tunnel for flap transfer is outlined high in the axilla (Fig. 44.3).
For both immediate and delayed breast reconstructions, the procedure begins with the patient supine. In immediate reconstruction, the mastectomy is performed generally through a skin-sparing pattern used in conjunction with the general surgeon. In delayed reconstruction, the mastectomy wound is opened and the mastectomy flaps are elevated to the margins of the defect. At this point in both situations, dissection is performed high in the axilla until the lateral margin of the LDMF is identified. By opening the mastectomy defect initially, eventual passage of the LDMF anteriorly to the mastectomy wound is facilitated, which eases closure of the back donor site after
the flap is elevated. This is opposed to packing the flap up into the axilla after flap elevation, which can crowd the closure of the back. In addition, by keeping most of the lateral margin of the mastectomy defect largely intact and communicating with the eventual back donor site high in the axilla, this important breast contour can be effectively controlled. Inadvertent release of the lateral margin of the breast is difficult to reconstruct with internal sutures; thus, it is far preferable to keep these attachments intact to smoothly form the lateral breast contour. The mastectomy wound is temporarily closed with staples and covered with an occlusive dressing.
the flap is elevated. This is opposed to packing the flap up into the axilla after flap elevation, which can crowd the closure of the back. In addition, by keeping most of the lateral margin of the mastectomy defect largely intact and communicating with the eventual back donor site high in the axilla, this important breast contour can be effectively controlled. Inadvertent release of the lateral margin of the breast is difficult to reconstruct with internal sutures; thus, it is far preferable to keep these attachments intact to smoothly form the lateral breast contour. The mastectomy wound is temporarily closed with staples and covered with an occlusive dressing.
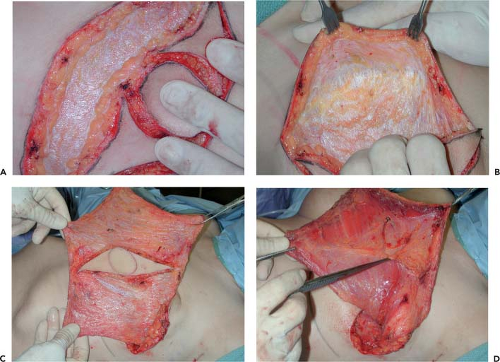
The drapes are removed, and the patient is repositioned to prepare for harvesting the LDMF. Flap elevation can be performed with the patient in the lateral decubitus position, but it is my preference to use the prone position for both unilateral and bilateral cases because positioning and appropriately padding the patient seem to be more easily accomplished. As the skin is incised, the more compact superficial layer of fat is divided down to the deep thoracic fascia. This fascia is then released around the periphery of the skin island. As the fascia is divided, the wound seems to spring open. With upward traction placed on the skin edges, the looser and less compact deep layer of fat pulls away from the underside of the fascia. Dissecting in this plane in all directions then preserves this deep layer of fat as it remains attached to the muscle. By adding fat to the flap in this manner, the soft tissue volume of the LDMF is increased (Fig. 44.4). Even if the thickness of the fatty layer is only 5 mm, over a 20 by 30 cm surface area, an additional 300 cc of volume are provided, which can add significantly to the ability of the flap to soften the contours of the reconstructed breast. This technique is referred to as the volume-added LDMF and is my preferred approach in patients of any body habitus. Even in very thin patients, this approach provides a ready plane of dissection even if only a modest amount of extra fat is added to the flap. With the use of this approach, the donor-site back skin flaps are of even thickness, which prevents contour irregularities from developing postoperatively. Dissecting below the fascia as described prevents inadvertent devascularization of the back flaps because they are essentially fasciocutaneous in design. Dissection proceeds in this plane in all directions and extends to the margins of the muscle. After the flaps have been developed, the deep
layer of fat is divided by angling through it toward the periphery in all directions. Superiorly, dissection proceeds beyond the muscle border to allow the deep fatty layer in this area to be included with the flap. In the superomedial corner of the dissection space, the obliquely oriented fibers of the trapezius muscle are identified. After this important landmark is isolated, the remainder of the dissection proceeds without difficulty. The value of this maneuver may not be evident in thinner patients because the margins of the muscle are usually readily identifiable; however, in heavier patients, it can be difficult to locate with certainty the exact anatomic relationships required for accurate flap dissection. By locating the fibers of the trapezius as described, the rest of the muscle dissection can be accurately performed. Superiorly, the deep layer of fat is divided and then peeled back to reveal the superior border of the latissimus, which is released all the way up into the axilla. It should be noted that in some patients, the fibers of the teres major will fuse with those of the latissimus and sharp dissection will be required to separate them. The muscle is then undermined and released from its attachments under the trapezius and then medially along the paraspinous fascia. Large crossing intercostal perforators are present midway through this elevation, and these vessels are controlled with silk ties. Inferiorly, the muscle is released from medial to lateral, and often a small cuff of muscle is left behind because it is not necessary to dissect completely down to the iliac crest to appropriately harvest the flap. At this point, the location of the serratus anterior becomes evident as it attaches to the scapula and crosses obliquely forward. The dense fascial attachment of the latissimus to the serratus is divided, and the subserratal fat pad is kept attached to the latissimus muscle to provide added bulk to the flap. Dissection then proceeds anteriorly between the serratus and the latissimus until the anterior border of the latissimus is released. Finally, the anterior inferior corner of the latissimus is released, with care being taken not to injure the dense attachments with the muscle fibers of the external oblique and the intercostals (Fig. 44.5). At this point, the muscle/fat flap is essentially completely elevated. In the axilla, the deep fatty layer is divided, and dissection proceeds along the muscle fibers until a communication with the mastectomy wound is created high in the axilla. Under the muscle insertion, the vascular pedicle is easily identified and preserved. The serratus branch is identified and left intact because it is not necessary to divide this vessel to allow adequate anterior transposition of the muscle. The dense clavipectoral fascia is divided because this attachment can hinder anterior transposition of the muscle. The latissimus muscle is fully detached from its attachments to the teres major and the overlying fat, and any remaining soft tissue attachments that might possibly restrict flap transposition are released and the flap is transferred to the mastectomy defect. The back wound is closed in layers over a suction drain brought out through the posterior axilla. During closure, quilting sutures attaching the underside of the flaps to the chest wall can be placed to help close down this potential space. In addition, at the level of the incision, quilting sutures can be used to help hold the soft tissue envelope of the back in position and prevent it from sliding over the chest wall. It
has been my observation that the incidence and severity of seroma formation are reduced as a result of placing these sutures.
layer of fat is divided by angling through it toward the periphery in all directions. Superiorly, dissection proceeds beyond the muscle border to allow the deep fatty layer in this area to be included with the flap. In the superomedial corner of the dissection space, the obliquely oriented fibers of the trapezius muscle are identified. After this important landmark is isolated, the remainder of the dissection proceeds without difficulty. The value of this maneuver may not be evident in thinner patients because the margins of the muscle are usually readily identifiable; however, in heavier patients, it can be difficult to locate with certainty the exact anatomic relationships required for accurate flap dissection. By locating the fibers of the trapezius as described, the rest of the muscle dissection can be accurately performed. Superiorly, the deep layer of fat is divided and then peeled back to reveal the superior border of the latissimus, which is released all the way up into the axilla. It should be noted that in some patients, the fibers of the teres major will fuse with those of the latissimus and sharp dissection will be required to separate them. The muscle is then undermined and released from its attachments under the trapezius and then medially along the paraspinous fascia. Large crossing intercostal perforators are present midway through this elevation, and these vessels are controlled with silk ties. Inferiorly, the muscle is released from medial to lateral, and often a small cuff of muscle is left behind because it is not necessary to dissect completely down to the iliac crest to appropriately harvest the flap. At this point, the location of the serratus anterior becomes evident as it attaches to the scapula and crosses obliquely forward. The dense fascial attachment of the latissimus to the serratus is divided, and the subserratal fat pad is kept attached to the latissimus muscle to provide added bulk to the flap. Dissection then proceeds anteriorly between the serratus and the latissimus until the anterior border of the latissimus is released. Finally, the anterior inferior corner of the latissimus is released, with care being taken not to injure the dense attachments with the muscle fibers of the external oblique and the intercostals (Fig. 44.5). At this point, the muscle/fat flap is essentially completely elevated. In the axilla, the deep fatty layer is divided, and dissection proceeds along the muscle fibers until a communication with the mastectomy wound is created high in the axilla. Under the muscle insertion, the vascular pedicle is easily identified and preserved. The serratus branch is identified and left intact because it is not necessary to divide this vessel to allow adequate anterior transposition of the muscle. The dense clavipectoral fascia is divided because this attachment can hinder anterior transposition of the muscle. The latissimus muscle is fully detached from its attachments to the teres major and the overlying fat, and any remaining soft tissue attachments that might possibly restrict flap transposition are released and the flap is transferred to the mastectomy defect. The back wound is closed in layers over a suction drain brought out through the posterior axilla. During closure, quilting sutures attaching the underside of the flaps to the chest wall can be placed to help close down this potential space. In addition, at the level of the incision, quilting sutures can be used to help hold the soft tissue envelope of the back in position and prevent it from sliding over the chest wall. It
has been my observation that the incidence and severity of seroma formation are reduced as a result of placing these sutures.
The patient is then rotated into the supine position, and the chest is prepped and draped, including the tops of the shoulders. This is an important nuance because meaningful evaluation of breast shape requires proper positioning of the shoulders, and the ability to see this position increases the accuracy of the procedure. The mastectomy wound is opened, and the LDMF is pulled completely into the mastectomy defect. At this point, the insertion of the muscle and the pedicle is more easily visualized than when the patient is prone. It is my preference to divide 90% of the insertion of the muscle because this allows an additional 10 to 12 cm of flap advancement into the mastectomy defect. Such advancement allows tension-free insetting and can facilitate proper orienting of the skin island, particularly in selected cases of delayed reconstruction. At the same time, keeping the anterior 10% of the insertion intact can prevent inadvertent traction from being placed on the pedicle with resultant vascular embarrassment of the flap. Finally, to lessen the degree of undesirable postoperative animation of the reconstructed breast, it is my practice to divide the thoracodorsal nerve. Since I have incorporated this maneuver into my technique, I have not observed a marked loss of volume in the reconstructed breasts over time, which would be the concern due to the expected atrophy that likely occurs in the muscle. Adding the deep layer of fat to the flap may assist in preventing this atrophy from becoming clinically significant. Of course, the nerve can be kept intact; however, the degree of movement that is sometimes present in the reconstructed breast can be disconcerting to some patients. For small to moderate-size breasts, the flap is then inset into the mastectomy defect by suturing the muscle and/or fat edge of the flap into the margin of the mastectomy defect superiorly, medially, and laterally. The tissue expander or implant is then inserted under the muscle, and the flap is inset along the inframammary fold (Fig. 44.6). If the fold was inadvertently lowered during the mastectomy, this maneuver can be effective in restoring the position of the fold to the desired level. For larger breasts, it is sometimes necessary to elevate the pectoralis major muscle to cover the upper pole of the
tissue expander or implant and to use the LDMF to cover the lower portion of the device. The two muscles are sutured together at the point where they overlap to complete the submuscular pocket. The skin island is inset, and final closure over a suction drain completes the procedure.
tissue expander or implant and to use the LDMF to cover the lower portion of the device. The two muscles are sutured together at the point where they overlap to complete the submuscular pocket. The skin island is inset, and final closure over a suction drain completes the procedure.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree