Fig. 17.1
(a) Operating room set up. (b) Trocar placement scheme. 1 Left subcostal, 2 Subxyphoid, 3 Optical, 4 Right subcostal, 5 Left anterior axillary line
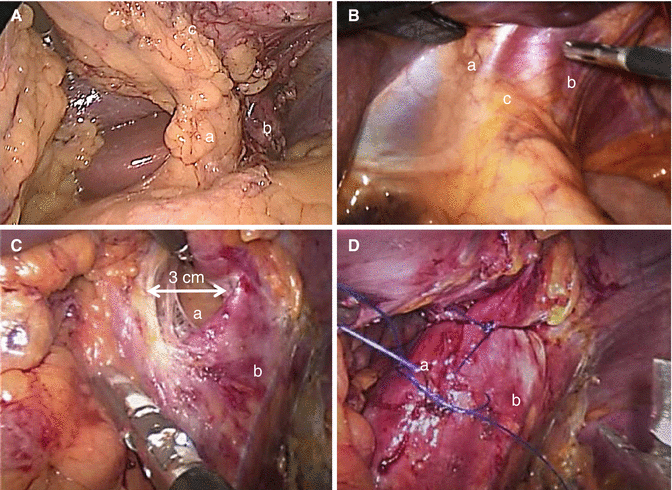
Fig. 17.2
(A) – The left gastric vessels (a) are visualized together with the left pillar (b). The posterior gastric wall (c) is completely free. The stomach is moved up like “the page of a book.” B – Finger print indentation on the peritoneum covering the hiatus orifice (c), right pillar (a), and left pillar (b). (C) Enlarged hiatus (a). Left pillar (b). (D) Hiatoplasty. Right pillar (a). Left pillar (b)
Anteriorly redundant Belsey’s fat pad may be resected in order to better visualize the gastroesophageal junction and the area where the stapler will be positioned. The posterior gastric wall must be freed by adhesions and occasionally it is necessary to divide posterior gastric vessels, when present, to ensure easy maneuvering of the stomach during resection. At the end of the mobilization the left gastric vessels and the left crus are clearly exposed and the stomach can be easily moved on its axis like “the page of a book” (Fig. 17.2A). Incomplete mobilization of the fundus may cause incomplete fundectomy determining an hourglass aspect of the stomach at the postoperative X-ray images and influencing long-term results.
The ablation of the GHR producing region of the stomach seems to be a crucial factor to ensure a proper functional result when considering EWL and comorbidities resolution: in several studies, it has been demonstrated that GHR concentration in the gastric mucosa increases from the corpus to the fundus. For this reason, the accurate fundectomy is a critical technical point [14, 15]. On the other hand, the accurate gastroesophageal junction mobilization by division of the short gastric vessels, of posterior gastric artery and of phrenic branches when present, in order to perform an ideal fundectomy may hamper the blood supply of this area and facilitate the onset of the gastric leak that occurs quite uniformly at the uppermost part of the suture line. Sound judgment must be used to balance the risks (leak) and the benefits (functional results).
Careful inspection of the hiatal area is performed after complete mobilization of the gastric fundus and of the posterior gastric wall. Enlarged hiatus and hiatal hernias must be identified. A 3 cm diameter of hiatal orifice is considered abnormal. As a practical rule in our clinical practice, exploration of the hiatal crura is indicated by a macroscopically evident fingerprint indentation of the diaphragm just above the esophageal emergence from the diaphragm (Fig. 17.2B). In these cases, the dissection of the hiatal crus is indicated and easily performed, because of the mobilized fundus, from the left approach (Fig. 17.2C). When present, the hernia sac and the gastroesophageal fat pad are dissected and reduced within the abdominal cavity. Retrogastric lipomas should be identified and removed to diminish the occurrence of the sliding of the gastric tubule into the mediastinum. A posterior hiatoplasty is performed. The hiatal crus defect is repaired with two or three interrupted nonabsorbable sutures between the right and the left diaphragmatic pillars (Fig. 17.2D) [16].
Once the stomach is completely mobilized, an oro–gastric tube is inserted by the anesthesiologist in order to calibrate the resection. A 48-French bougie is pushed down possibly through the pylorus and placed against the lesser curvature. The use of tube sizes from 30- to 60-French bougie is reported in the literature. There is evidence that smaller sizes of bougie (<40 Fr) correlate to a significant increase of gastric leaks [17–19]. On the other hand, it is not clear if the bougie size can significantly affect the capacity of the residual stomach and the postoperative weight loss. In our experience, the capacity of the gastric remnant was dependent not by the bougie size but by other technical points: placement of the stapler well against the bougie, complete gastric fundus dissection, and lateral traction of the gastric walls during the resection.
Gastric resection is performed using a linear stapler. The stapler is applied alongside the calibrating bougie. Because of the decreasing thickness of the gastric wall from the antrum to the corpus and fundus, the cartridges must be chosen accordingly. The staple height used by us is 4.4 or 4.1 mm near the antrum and 3.8 or 3.5 mm on corpus and fundus. In revisional surgery cases, the presence of scar tissue indicates the use of higher staples. The stapler is inserted through the right subcostal trocar for the first and second firing and then through the left subcostal trocar. Occasionally, it can be inserted through the optical trocar and the camera moved to the left subcostal trocar. The stapler branches must be coplanar to the gastric walls to avoid tubule twisting.
Before closing and firing the staplers, the anterior and posterior gastric walls are fully stretched by two graspers precisely positioned on the greater curve and moved along it (Fig. 17.3A).
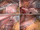
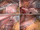
Fig. 17.3
(A) Traction on the gastric walls by two graspers (a, b) on the greater curvature. The suture line (c) is alongside the bougie. (B) The last stapler is fired 1.5 cm from the angle of His. (C) The final end of the buttressing membrane (b) is fixed to the left pillar (a) by two nylon stitches (arrows). (D) Final view. Several metal clips complete the hemostasis on the suture line
At the incisura angularis, the stretching is somewhat loosened to avoid a functional stricture that may occur at this level (Fig. 17.4C). The last cartridge is fired 1–2 cm away from the angle of His so that the staple line does not fall in the “critical area” (Fig. 17.3B). The staple line is reinforced by buttressing with absorbable polymer membrane (Seamguard, Gore). After moving the stomach specimen away from the left subcostal space, the final end of the membrane is fixed with two nonabsorbable sutures to the left pillar to avoid sliding of the stomach tubule into the mediastinum (Fig. 17.3C). At the end of the procedure, the staple line is meticulously checked and bleeding spots are treated by hemostatic clips or stitches (Fig. 17.3D). A nasogastric tube is positioned in the gastric remnant and a methylene blue dye test is routinely performed to check the sealing of the staple line and to evaluate the residual gastric capacity, usually 60–80 ml.
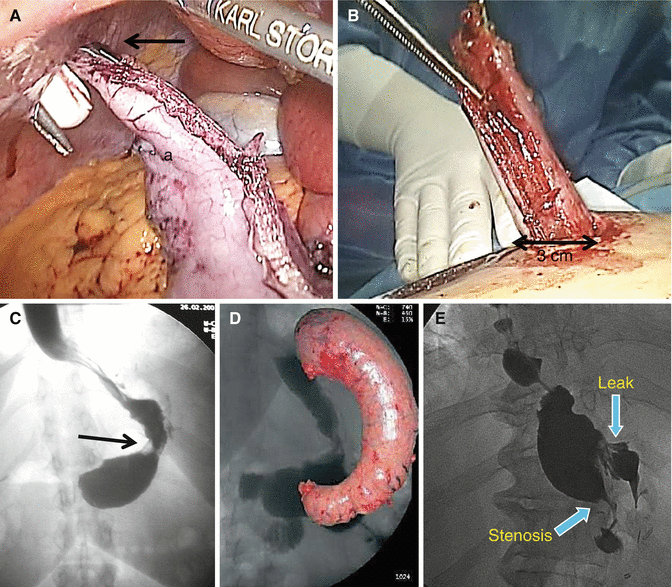

Fig. 17.4
(A) Specimen extraction. Grasper (arrow) on the antrum end of the gastric specimen (a). (B) Extraction through a 3 cm enlarged trocar access. (C) Functional stenosis at the middle third (arrow) of the gastric tubule. The prestenosis segment is not dilated. (D) Gastric specimen. In the background: normal post-op X-ray aspect of the gastric tubule. (E) Post SG upper GI contrast X-ray: organic stenosis and gastric leak. Dilation of the prestenosis segment
The specimen is extracted by grabbing its distal end with a grasper (Fig. 17.4A) and it is easily brought out of the abdominal cavity through the slightly enlarged right subcostal access without the need of retrieval bags or endo loops and taking care not to open the specimen during these maneuvers (Fig. 17.4B). A gauze soaked in povidone–iodine solution (betadine) is left for 1–2 min on the retrieval site to avoid infection of the wound [20].
When gallbladder stones are present, cholecystectomy is routinely performed at completion of the SG procedure. The same trocars are used. Occasionally, in complicated cases, an additional 5 mm trocar is added 5 cm laterally to the right subcostal trocar. Drains are not routinely placed and the nasogastric tube is removed at the end of the procedure.
17.4 Postoperative Management
Patients are mobilized on the same day of the operation and maintained with intravenous fluid therapy, proton pump inhibitors, and analgesics. Subcutaneous low-molecular-weight-heparin is administered 6 h after surgery and is continued for 2 weeks. Short-term antibiotic therapy is added. Upper gastrointestinal contrast (Gastrografin) study is performed on the second or third postoperative day. Afterward patients are started on liquid diet and discharged on the fourth postoperative day.
Soft diets with mashed and soft foods are prescribed for 4 weeks after surgery. One month after surgery, patients resume normal diet with the advice of adding one type of food at a time, meat may take longer to be well tolerated. Five small meals are suggested.
Postoperative follow-up is performed at 1, 3, 6, 12, 18, and 24 months after the operation, annually afterward. Controls involve Physical examination, blood tests (including vitamin B12, folate, serum iron, calcium and vitamin D serum levels), upper gastrointestinal contrast (at 1st month and 1st year), liver ultrasound (at sixth month). Endoscopic check is mandatory 2 years after the operation in all patients.
Oral proton pump inhibitors and urso-deoxy-cholic acid for 6 months and multivitamin tablets for 1 year are prescribed.
17.5 Complications
The postoperative mortality rate varies from 0.1 to 0.5 % and the postoperative morbidity rate ranges from 0.0 to 12 % [5, 10, 12]. Early diagnosis is the most important factor to ensure a positive solution of complications. Treatment is often challenging and should be managed in bariatric centers and by dedicated medical teams. Operative treatment is required only in few selected cases.
Bleeding: Significant hemorrhage occurs in 1.1–8.7 % of cases. Most frequently, bleeding occurs within the first 24–48 h and originates from the staple line [5, 21, 22]. Other sites of bleeding are the gastroepiploic or short gastric vessels divided during the mobilization of the stomach, the trocar accesses, hepatic or splenic injuries. Almost always, the bleeding is into the abdominal cavity, rarely determines hematemesis or melena. Once the hemodynamic parameters are stable, CT-scan is mandatory in order to define the bleeding site and to quantify the hemoperitoneum. In case of hemorrhage from the staple line, the CT images show a hematoma close to the gastric remnant. The treatment is interventional radiology or, in fewer instances, open or laparoscopic surgical exploration. Suture line reinforcement has significantly reduced the occurrence of this complication. Bleeding from the staple line increases the risk of gastric leak.
The two major most debated complications are staple line leak and gastroesophageal reflux disease (GERD). Three main factors should be considered in their pathophysiology: intraluminal pressure, intra-abdominal pressure, and critical vascularization of the gastroesophageal junction.
Leak: Staple line leaks represent the most dangerous and life-threatening complication after LSG, with an incidence between 0 and 7 %. Leaks occur mostly during the first post-op week (early leaks) and, in fewer instances, in the 40 days postoperative period (late leaks) [10, 11]. The most common location of leaks is just below the esophageal gastric junction. Causative factors to be considered in its genesis are: high intragastric pressure, thin wall of the gastric fundus, transitional vascularization (esophageal arteries system above, gastric arteries system below) on the left side of the esophageal-gastric junction (“critical area”) causing ischemia [13]. Technical factors include small bougie size and tight sleeves, heat from electrocautery or other energy sources during dissection, or hemostasis which may determine gastric injury.
Some technical details may lessen the incidence of leaks: the final portion of the line of resection should avoid the “critical area” remaining 1–2 cm laterally to the angle of His; the gastric resection should be more loose at the incisura angularis, the most frequent site of stenosis.
The treatment of leaks is challenging and different managements have been reported. In our experience, operative treatment is reserved only in patients with hemodynamic instability and signs of acute peritonitis. Peritoneal toilet and proper drainage are recommended. Attempts to repair the fistula are contraindicated: recurrence occurs in most cases. An unsuccessful control of the leak, at occasion, may require total gastrectomy or creation of a Roux limb. In the vast majority of patients, nonoperative management by percutaneous CT-guided drainage, alone or in combination with stent placement and enteral nutrition, is a safe and effective treatment for suture line leaks [23].
In an attempt to reduce the incidence of staple line bleeding and/or leak in recent years several different techniques have been adopted to reinforce the staple line during LSG. The routine use of reinforcement of staple line seems to reduce the complications rate. At present, the main options are: oversewing the staple line with a running or inverting absorbable suture, buttressing the staple line with absorbable materials, bovine pericardium strips or porcine small intestine submucosa, applying fibrin glue or hemostatic agents on the staple line [24, 25]. In all published studies, staple line buttressing was reported to reduce significantly the incidence of bleeding; however, the preventive effect on gastric leak was less evident. In a recent review of 88 papers reporting on 8,920 patients, leak rate in LSG was significantly reduced by buttressing the staple line with absorbable polymer membrane compared to over sewing, bovine pericardium strips, or no reinforcement [26].
Stenosis: Stenosis is reported with an incidence ranging from 0.2 to 4 % and usually occurs at the corpus-antrum transition zone (incisura angularis) of the gastric tubule. At this site transient functional stenosis (Fig. 17.4C), due to dysmotility because of the muscular layers section, may occur causing high intragastric pressure and so favoring leak occurrence. Mechanical stenosis, causing significant and lasting dysphagia and vomiting, can be determined by an incorrect orientation of the stapler tip during the resection or subsequent to an imbrication of the staple line. Twisted sleeve may cause symptomatic stenosis. An upper gastrointestinal (GI) contrast study is indicated to confirm the gastric outlet obstruction (Fig. 17.4E). Endoscopy has an important role in terms of diagnosis and treatment. Repeated endoscopic dilations are the first therapeutic approach. Placement of endoscopic stents should be considered as alternative solution. In case of persistence of symptoms with nutrition problems, patient’s reoperation should be considered [27]. Conversion to Roux-en-Y gastric bypass is a valid therapy. Laparoscopic seromyotomy of the stenotic tract (stricturoplasty) has also been proposed [28].
Hiatal hernia, GERD: In the Fourth International Consensus Summit on SG held in 2012, there was a general agreement that when a hiatal hernia is present it should be repaired at the time of the bariatric procedure. In the same Consensus postoperative GERD was the most frequently reported complication with a mean incidence of 7.9 % [12]. However, the clinical and pathophysiological relationship between SG and GERD is still debated. In some series a postoperative improvement of GERD symptoms has been reported while in other series a worsening has been registered [29–32




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







