Introduction
The mobilization of soft tissues to reconstruct cutaneous operative wounds is more than just an exercise in geometry.1 Instead, reconstructive procedures involve the manipulation of biologic tissues with the primary purpose being an approximation of the preoperative state of “normalcy.” The mystical attainment of an invisible scar and a complete restoration of the presurgical condition is a worthwhile goal that can be closely approximated—even if perfection is unattainable. The degree to which a minimally perceived result is approached is dependent on a number of biologic factors beyond the surgeon’s control. These include the patient’s age and general health, the long-term use of certain medications, whether or not the patient smokes, and a number of uncontrollable cutaneous variables such as skin thickness, sebaceous quality, pigmentation, elasticity, actinic damage, prior surgical scarring, and individual variations in scar formation.
Many mechanical tissue parameters are amenable to manipulation. The interaction of the intrinsic biologic properties and the mechanical operations performed on tissues may be aptly described as the biomechanical aspects of wound closure.2–4 The biology of tissue is a major determinant of its ability to move. This is readily observed in the skin tension lines on the face, where closure in one direction is facile, and perpendicular closure is challenging.5 The response of tissue during reconstruction involves both intrinsic biologic and mechanical properties and the physics of forces and motion. Knowledge of the mechanics of reconstruction augments the surgeon’s ability to design an appropriate wound closure. The limiting biologic properties dictate the available menu of reconstructive designs available for wound closure. Concepts that seem simple and intuitive often have hidden complexity that in select instances become important in optimizing the final closure result. The goals of a successful reconstruction procedure can be arbitrarily divided along biologic and mechanical lines, and each plays an important role in a successful reconstruction.
The mechanical plan of tissue movement is designed to achieve a closure: (1) under minimal tension; (2) without distortion of critical anatomic structures and landmarks such as the lip, nasal rim, eyebrow, and hairline; (3) using skin of matching pigment and texture to the affected region; and (4) with consideration to optimal placement of scars along cosmetic unit junctions. The mechanical reconstructive design is therefore implemented in an attempt to reestablish an aesthetic and functional baseline.
The biologic considerations to tissue movement involve: (1) maintenance of the viability of mobilized tissues; (2) preservation of sensory and motor innervation; (3) appropriate mobilization of tissues to allow for wound closure; and (4) prevention of morbidity such as hematoma, infection, and dehiscence. In order to understand macro-biomechanical concepts, an anatomic model for facial surgery will first be introduced.6 This will be followed by a discussion of the manipulations used to modulate or decrease tension, redistribute tension vectors, and to reposition redundant tissue. Jointly, these topics are the crux of clinical biomechanics, as they pertain to successful adjacent tissue transfers.
BIOANATOMY OF TISSUE MOVEMENT
Rather than reiterating classical anatomy,7,8 biomechanics is better understood by introducing, for the face, a heuristic, clinically applicable model of structural organization. The three units to be introduced are fascia, vasculature, and nerve distribution. The anatomic patterns are repeatedly underscored in order to emphasize the biologic implications and their influences on tissue movement.
Fascia
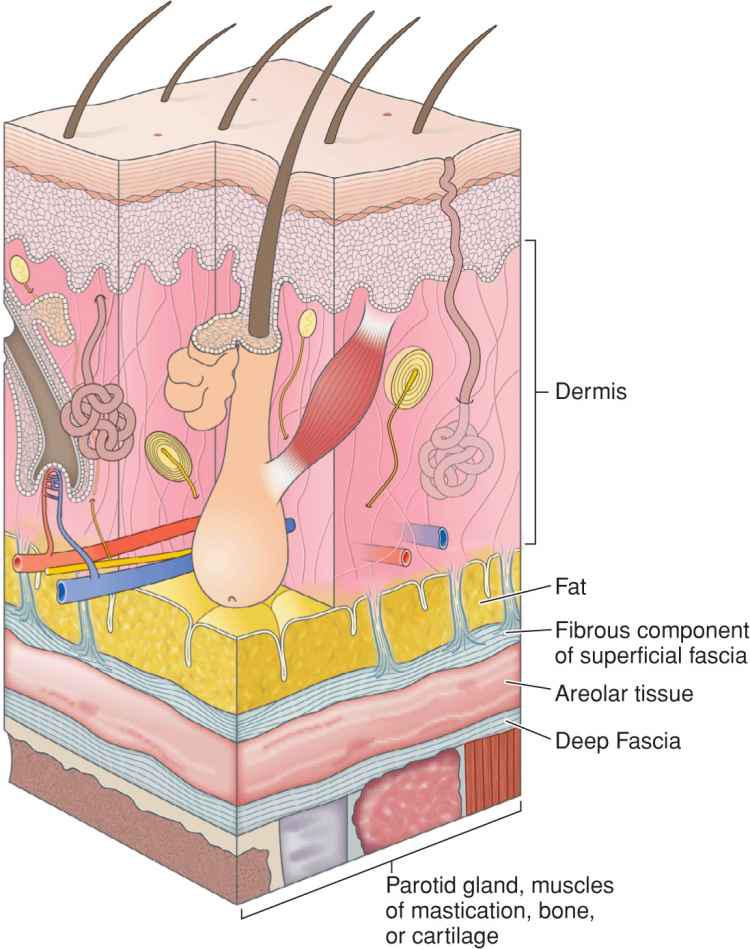
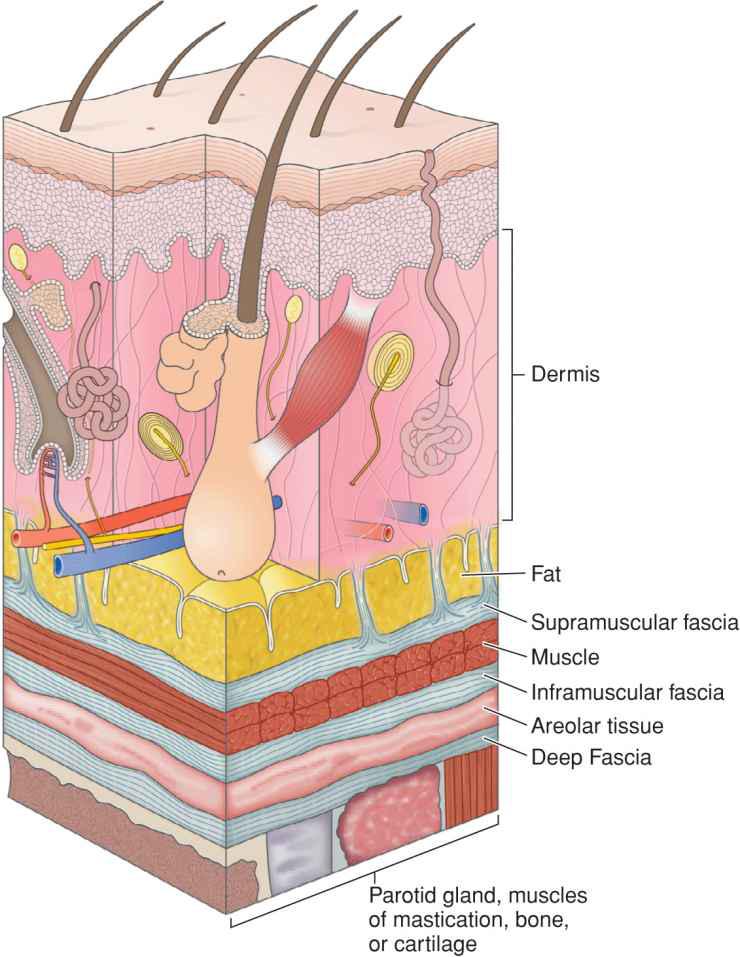
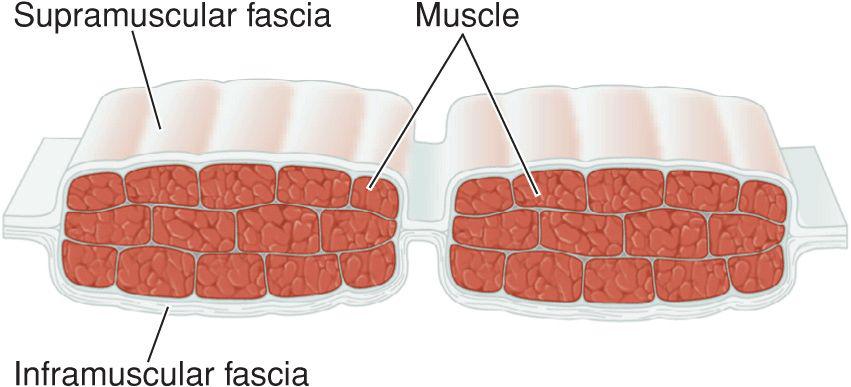
Fascia provides the structural skeleton for the anatomic organization of vascular and neural structures.9–11 The fascia of the face is nosologically divided into a deep and a superficial component12 (Fig. 1.1). Superficial fascia is composed of a fatty subcutaneous portion and a deeper fibrous layer that appears to be derived from the interlobular septae of the fat. The fibrous component of the superficial fascia integrates and connects the various muscles of facial expression. Where there is an absence of facial musculature, the fibrous component is a thick, nonstretchable membrane. Clinically, this is observed as the galea of the scalp, as the superficial temporal fascia, and as the superficial musculoaponeurotic system (SMAS) of the cheek. In the presence of the facial musculature, the fibrous portion of the superficial fascia bifurcates to envelope the muscle (Fig. 1.2). The component that splits superficial to the muscle is typically thin and mobile, but the deep component retains the thick, inelastic quality of the fibrous fascia of the nonmuscular areas. This network of fibrous fascia interlinking and enveloping the facial musculature integrates and coordinates complex facial movements (Fig. 1.3).
Figure 1.1 The superficial fascia has two layers separated by loose areolar tissue. The superficial fascia attaches to the overlying adipose through small fibrous attachments. The deep fascia envelopes the facial musculature and parotid gland and is more densely adherent
Figure 1.2 The fibrous portion of superficial fascia envelopes the muscles of facial expression. The supramuscular fascia is thin while the deep fascia is thick and inelastic
Figure 1.3 The fibrous portion of superficial fascia envelopes the muscles of facial expression, interconnecting them for coordination of complex movements
The deep fascia of the face is separated from the superficial fascia by loose, relatively avascular areolar tissue. Facial deep fascia covers cartilage, bone, muscles of mastication, and visceral structures. Similar to the superficial fascia, the deep fascia is a continuous sheet. The nomenclature is altered as it involves various structures, and therefore, deep fascia encompasses the perichondrium, periosteum, temporalis muscle fascia, and parotid-masseteric fascia.
Significant biomechanical consequences result from the incorporation of fascia into mobilized tissues. Mechanically, the fibrous component of the superficia fascial inhibits tissue mobility and prevents the normal elasticity of the skin from contributing to the closure process. Clinically, this situation is exemplified on the scalp and forehead. Undermining is readily accomplished in the deep avascular plane beneath the galea. However, despite extensive undermining, little tissue mobility is gained. The fascia in this area simply does not stretch.
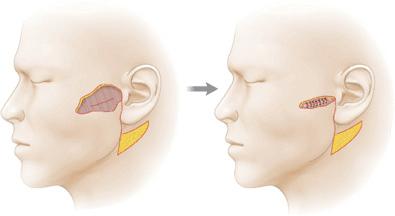
Fascia may be used mechanically for benefit. Plication of the fibrous component of the superficial fascia can be used to relieve closure tension on the dermis. The fascia of the cheek and neck is frequently plicated in rhytidectomies and reconstructive cheek closures to minimize skin closure tensions13 (Fig. 1.4). Because of the attachment of the dermis to fascia through interlobular septae, skin is moved without being under tension itself. As an avascular structure with few nutritional requirements, the fibrous component of the superficial fascia is able to bare significant force without vascular compromise. Similarly, the superficial fascia can be anchored to the deeper fascia, thus preventing tension on a free margin such as the eyelid.14
Figure 1.4 The SMAS may be plicated to achieve reduction in surface (dermal) wound closure tension
Tension redistribution is also relevant when using the deep fascia in a repair. As noted earlier, deep fascia may be used as an immobilizing structure or anchor to which tissue may be fixed to prevent pull and distortion. Suspension sutures may be placed between mobilized tissue and deep fascia, especially the periosteum, to prevent tension on anatomic landmarks and moveable structures. In the malar area, for example, tissue may be suspended to the infraorbital rim periosteum to prevent vertical tension and ectropion. Flaps from the temple and cheek may be tacked to the lateral orbital rim periosteum to prevent tension on the lateral canthus and lateral ectropion. Therefore, although fascia may mechanically inhibit tissue elasticity, it may be selectively used to relieve skin tension and guide wound closure.
Vasculature
The axial vasculature of the face consists of named branches and their associated angiosomes. An angiosome is a three-dimensional tissue block consisting of muscle, fascia, subcutaneous fat, and skin that is supplied by a particular source artery. On the face there are 13 angiosomes corresponding to larger arterial branches.15 The named axial arteries such as the facial artery, superficial temporal artery, infraorbital artery, supratrochlear artery, and supraorbital artery branch widely and anastomose broadly to provide a rich arterial supply to the face.
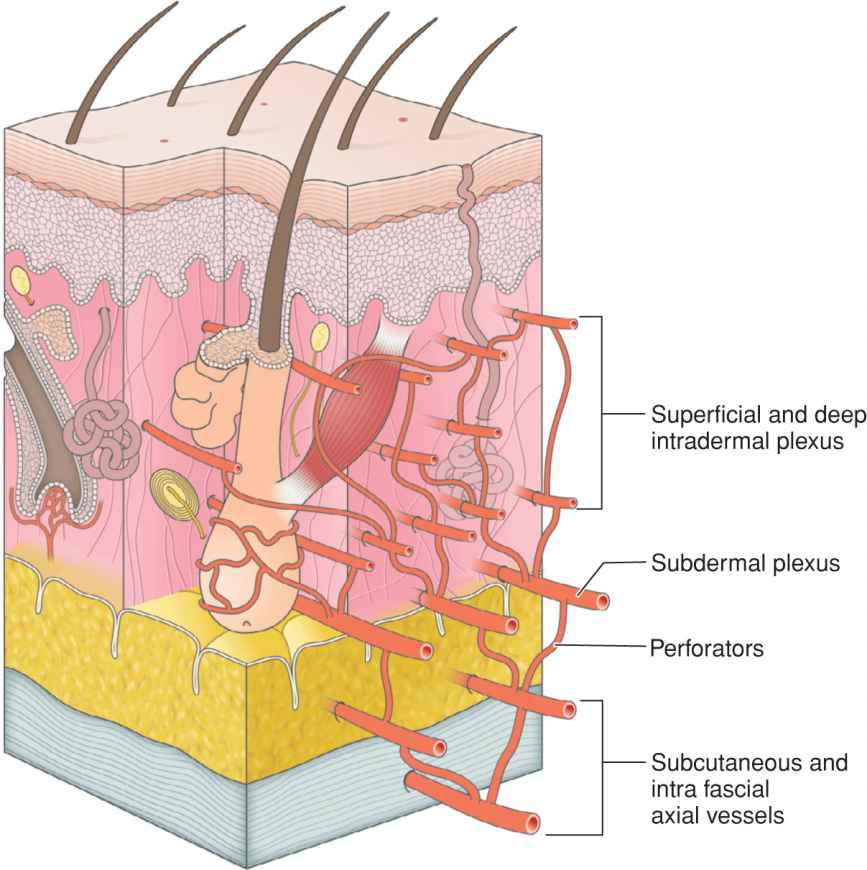
Facial vascular patterns may be organized by vessel caliber, depth, and orientation16–22 (Fig. 1.5). The named arteries branch and ascend to run in within the superficial fibrous fascia, from which point they give off numerous vertically oriented vessels, which then ascend into the adipose tissue where they branch and interconnect to form a subcutaneous vascular plexus. The subcutaneous plexus is in turn connected to the deep dermal vascular plexus which is then connected to the superficial dermal plexus. The superficial and deep intradermal plexi are composed of a microvascular network that is usually unable to support tissue viability when a flap is performed. The shallowest substantial vascular supply that can support an adjacent tissue transfer is the subdermal vascular plexus. The vessels of the subdermal plexus run horizontally within the superficial subcutaneous tissues. They are preserved by leaving a layer of adipose on the undersur-face of any adjacent tissue transfer.
Figure 1.5 Cutaneous and subcutaneous vasculature is composed of horizontally and vertically oriented networks with the caliber of vessels largest where they run within or just deep to the fascia
In most facial reconstructions, the redundancy of the subdermal plexus allows for a reliable blood flow; however, incorporation of vessels from the deep plexus can be needed in certain instances due to flap design, flap tension, or underlying host factors such as heavy smoking. Mechanical planning must always be coupled with an awareness of how tissue viability is to be maintained.
Nerves
Neural input is unimportant to flap survival, but flap design and actuation should take into consideration the underlying nerves, as neural function is of crucial importance to host biology and function.
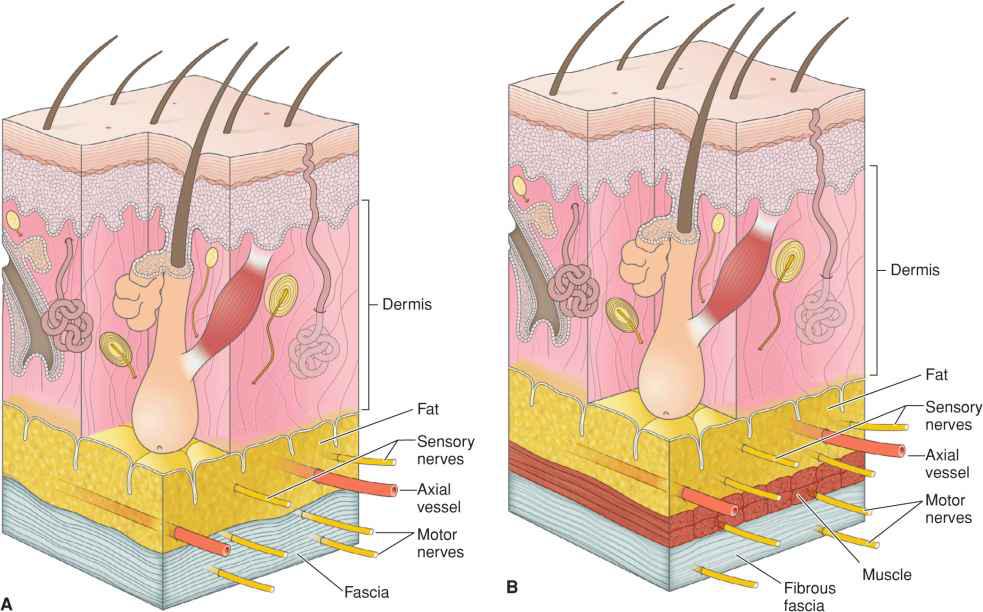
Two patterns of neural organization apply: sensory innervation and motor innervation. Sensory innervation of the face is derived primarily from branches of the trigemina nerve and the first several cervical nerves. After leaving their foramina of origin, the trigeminal nerve branches ascend to the level of the superficial fascia where they run just above the fibrous component of the superficial fascia or within the subcutaneous tissues (Fig. 1.6). In areas where the SMAS envelopes facial musculature, the sensory nerves are within the thin supramuscular component and are often accompanied by small axial vascular branches creating neurovascular bundles. Small branches of these nerves intermittently ascend to innervate portions of the overlying skin. Therefore, undermining at any level is capable of causing sensory denervation.
Figure 1.6 (A) Sensory nerves are usually paired with axial vessels where they run as neurovascular bundles within the thin superficial fascia. Motor nerves run within the deeper fibrous component. (B) Where facial muscle exists, sensory nerves run superficial to the muscle, whereas motor nerves run deep to muscle
Several areas are particularly prone to sensory disruption. The forehead is richly innervated by the supratrochlear and supraorbital nerves. While these nerves emerge deeply from bony foramina, they ascend quickly to run over the surface of the frontalis muscle. Incisions on the forehead, especially those that are horizontal, risk disruption of these nerve branches. While the most common eventuality of such disruption is a band of numbness on the forehead and anterior scalp, permanent numbness and neuralgia can result. Another area in which sensory disruption is common is in the periauricular region, where the large greater auricular nerve lies above the fascia just behind and beneath the earlobe. The auriculotemporal nerve is vulnerable just superior to and anterior to the tragus. Other sensory nerves tend to lie more deeply beneath fascial layers.
More important to host function is the integrity of the motor nerves supplying the muscles of facial expression. Motor innervation is derived primarily from the branches of the facial nerve.23–26 The facial nerve is protected by the parotid gland on the lateral face. After leaving the parotid fascia, the branches of the facial nerve ascend to the level of the superficial fascia where they travel within the fibrous component. For that reason, undermining above the fibrous component of the superficial fascia and within the subcutaneous fat will rarely lead to motor nerve injury. However, careless incisions and/or undermining can easily damage these nerves. As the fibrous component envelopes the facial muscles, the nerves stay within the thick deep portion to produce appropriate innervation.
As a general rule, undermining at a level to include the subdermal plexus in mobilized tissue is without risk. Inclusion of deeper axial vessels may prove hazardous over zones where branches of the facial nerve traverse. The frontal and marginal mandibular divisions are at particular risk, because of their shallow locations and the paucity of interconnecting anastomoses.27,28
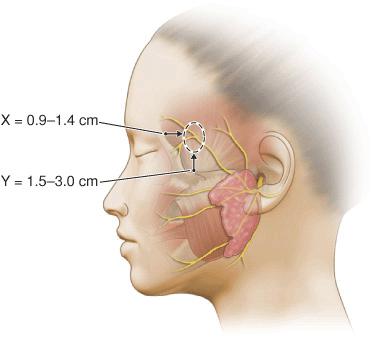
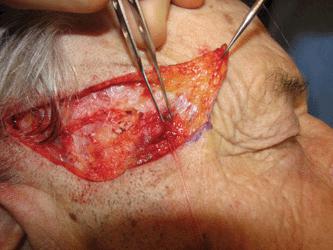
Particular attention must be paid to the frontal branch of the facial nerve.29,30 As the nerve ascends over the zygomatic arch, the superficial fascial complex is elevated closer to the surface and the fatty layer thins. Thus, although the pattern relationship is constant, the nerve is more exposed. While some texts have listed the nerve as running above the fascia, the nerve does lie within the innominate fascia just deep to the SMAS over the zygomatic arch and then ascends just slightly more superficially to lie within the superficial temporal fascia. This relationship has been exquisitely demonstrated by cadaver dissection.31 (Fig. 1.7). Incisions and undermining in this area, which stay above the fascia, are safe (Fig. 1.8). Coincidentally, this area harbors the temporal artery and large branches thereof (Fig. 1.9). They can serve as a marker for the depth at which excision and undermining expose the nerve to injury.
Figure 1.7 Illustration depicting the fascial transition zone (dotted circle) where the frontal branches transition from the innominate fascia to the superficial temporal fascia. The x-axis measurement was the distance posterior to the lateral orbital rim and the y-axis measurement was the distance superior to the upper border of the zygomatic arch (Reproduced with permission from Agarwall CA, Mendenhall SD, Foreman KB, et al. The course of the frontal branch of the facial nerve in relation to fascial planes: An anatomic study. Plast Reconstr Surg 2010;125:532-537. Copyright Wolters Kluwer Health.)
Figure 1.8 The frontal branch of the facial nerve is seen as it courses just beneath the nick created in the superficial fascia
Figure 1.9 The temporal vessels run just beneath the subcutis in the superficial fascia. The vessels run shallow to the frontal branch of the facial nerve. They are readily identified and staying above them when undermining provides a measure of safety
Bioanatomy is therefore used to facilitate successful tissue movement while preventing host morbidity. Fascial structure is an organizing framework for conceptualizing vascular and neural relationships. Fascia may be used mechanically to decrease tension forces in the skin and to immobilize critical structures. Knowledge of vascular and neural anatomy is important in the design of adjacent tissue movement in order to maintain viability and avoid morbidity.
MECHANICS OF TISSUE MOVEMENT
Adjacent tissue transfer involves the planned manipulation of closure force vectors to minimize the tension of wound closure and prevent anatomic distortion. This goal is accomplished by considering three variables: (1) tension reduction, (2) tension redistribution, and (3) dog-ear manipulation.
Tension Reduction
Low closure tension is the desirable choice to prevent tissue ischemia and necrosis, to avoid anatomic distortion, to minimize the spread of a scar, and to avoid wound dehiscence.32, 33 Increased closure tension reproducibly reduces blood flow, alters repair viabilty, and may lead to flap necrosis.34 Appropriate undermining can substantially reduce closure tensions;35, 36 however, a common misconception is that progressive undermining uniformly diminishes closure tension.37–39 In order to truly increase skin mobility, the tissue to be moved must be effectively separated from whichever structures are imposing the restriction. Effective undermining is more important than extensive undermining. Skin stretchability may be inhibited by direct or indirect attachment to the fascia, muscle, and bone. The mechanism of restriction is site specific and dependent on the particular anatomic organization.40
The scalp, for example, is tightly bound to the inelastic fibrous superficial fascial component termed galea, through the interlobular septae of the fat.41 The fascia is then attached to the bony supraorbital ridge, anteriorly, and occiput, posteriorly. This further inhibits mobility. If undermining is performed in the typical subgaleal avascular plane, the major restrictive component persists and significant laxity is not achieved. Movement may be partially gained by carrying undermining over the supraorbital ridge or nuchal crest, detaching the tissue from the bony restriction. Adverse consequences include eyebrow elevation anteriorly (desirable in the forehead lift) and potential damage to neurovascular bundles anteriorly and posteriorly. Scalp flaps may also be raised (with care) above fascia, thus diminishing the effect of galeal restraint (Fig. 1.10).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree