Innate and Adaptive Immunity in the Skin: Introduction
|
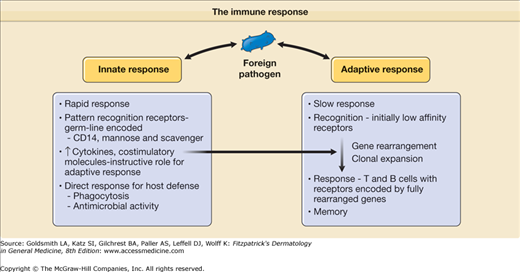
The human immune system is comprised of two distinct functional parts: (1) innate and (2) adaptive. These two components have different types of recognition receptors and differ in the speed in which they respond to a potential threat to the host (Fig. 10-1). Cells of the innate immune system, including macrophages and dendritic cells (DCs), use pattern recognition receptors encoded directly by the germ line DNA, respond to biochemical structures commonly shared by a variety of different pathogens, and elicit a rapid response against these pathogens, although no lasting immunity is generated. In contrast, cells of the adaptive immune system, T and B lymphocytes, bear specific antigen receptors encoded by rearranged genes, and in comparison to the innate response, adaptive immunity develops more slowly. A unique feature of the adaptive immune response is its ability to generate and retain memory; thus, it has the capability of providing a more rapid response in the event of subsequent immunologic challenge. Although the innate and adaptive immune responses are distinct, they interact and can each influence the magnitude and type of their counterpart. Together, the innate and adaptive immune systems act in synergy to defend the host against infection and cancer. This chapter describes the roles of the innate and adaptive immune response in generating host defense mechanisms in skin.
Innate Immune Response
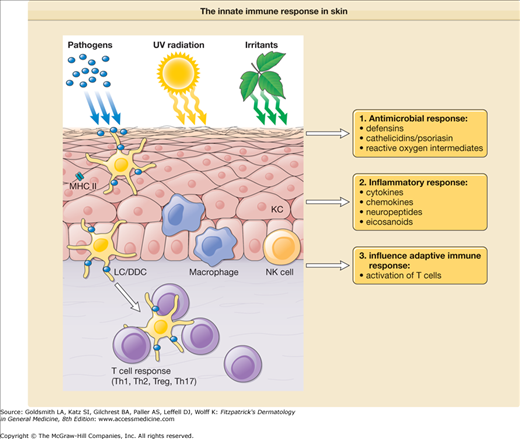
Immune mechanisms that are used by the host to immediately defend itself are referred to as innate immunity. These include physical barriers such as the skin and mucosal epithelium; soluble factors such as complement, antimicrobial peptides, chemokines, and cytokines; and cells, including monocytes/macrophages, DCs, natural killer cells (NK cells), and polymorphonuclear leukocytes (PMNs) (Fig. 10-2).
Figure 10-2
The innate immune response in skin. In response to exogenous factors, such as foreign pathogens, ultraviolet (UV) radiation, and chemical irritants, innate immune cells [granulocytes, mononuclear phagocytes, natural killer (NK) cells, keratinocytes] mount different types of responses including (1) release of antimicrobial agents; (2) induction of inflammatory mediators, such as cytokines, chemokines, neuropeptides, and eicosanoids; and (3) initiation and modulation of the adaptive immune response. DDC = dermal dendritic cell; KC = keratinocyte; LC = Langerhans cell; MHC II = major histocompatibility complex class II; Th1 = type I T cells; Th2 = type II T cells; Th17 = type 17 T cells; T reg = regulatory T cells.
Our present understanding of innate immunity is based on the studies of Elie Metchnikoff who, in 1884, published studies on the water flea Daphnia and its interaction with a yeast-like fungus.1 He demonstrated that cells of the water flea, which he termed “phagocytes,” were attracted to and engulfed the foreign spores, which were subsequently “killed and destroyed.” Thus, Metchnikoff described the key direct functions of cells of the innate immune system: (1) rapid detection of microbes, (2) phagocytosis, and (3) antimicrobial activity. In addition to this direct role in host defense, the innate immune system has an indirect role in instructing and determining the type of adaptive T and B cell responses. Finally, by inducing inflammation, the innate immune response can also induce tissue injury.
Physical structures prevent most pathogens and environmental toxins from harming the host. The skin and the epithelial lining of the respiratory, gastrointestinal, and the genitourinary tracts provide physical barriers between the host and the external world. Skin, once thought to be an inert structure, plays a vital role in protecting the individual from the external environment. The epidermis impedes penetration of microbial organisms, chemical irritants, and toxins; absorbs and blocks solar and ionized radiation; and inhibits water loss (see Chapter 47).
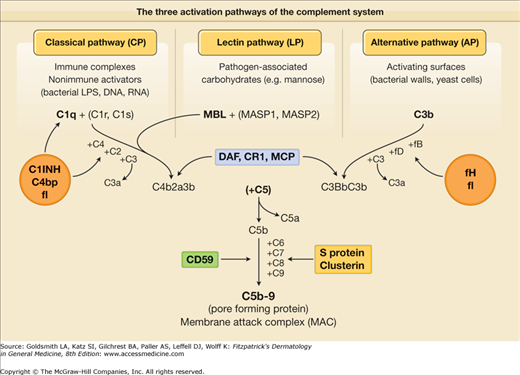
(See eFig. 10-2.1; see also Chapter 37). One of the first innate defense mechanisms that awaits pathogens that overcome the epithelial barrier is the alternative pathway of complement. Unlike the classical complement pathway that requires antibody triggering, the lectin-dependent pathway as well as the alternative pathway of complement activation can be spontaneously activated by microbial surfaces in the absence of specific antibodies (see eFig. 10-2.1). In this way, the host defense mechanism is activated immediately after encountering the pathogen without the 5–7 days required for antibody production.
eFigure 10-2.1
The three activation pathways of the complement system. Soluble proteins are shown in red, membrane-associated proteins in green. DAF = decay-accelerating factor; LPS = lipopolysaccharide; MASP = MBL-associated serine protease; MBL = mannose-binding lectin; MCP = membrane cofactor protein; C = complement; C1 INH = C1 inhibitor; C4bp = complement 4b binding protein; f = factor.
(See also Chapter 102).
![]() The skin is a rich source of neuropeptides, including neurotransmitters [e.g., calcitonin gene-related peptide (CGRP), substance P, somatostatin] and neurohormones (see Chapter 102). The inhibitory effects of CGRP and substance P on Langerhans cell (LC) antigen presentation function are discussed later. The neurohormone proopiomelanocortin (POMC) is produced by the pituitary gland as well as by a number of cell types, including keratinocytes.
The skin is a rich source of neuropeptides, including neurotransmitters [e.g., calcitonin gene-related peptide (CGRP), substance P, somatostatin] and neurohormones (see Chapter 102). The inhibitory effects of CGRP and substance P on Langerhans cell (LC) antigen presentation function are discussed later. The neurohormone proopiomelanocortin (POMC) is produced by the pituitary gland as well as by a number of cell types, including keratinocytes.
Antimicrobial peptides serve as an important evolutionarily conserved innate host defense mechanism in many organisms. They typically are positively charged and are amphipathic, possessing both hydrophobic and hydrophilic surfaces. The antimicrobial activity of these peptides is thought to relate to their ability to bind membranes of microbes (through their hydrophobic surface) and form pores in the membrane, leading to microbial killing. There are numerous antimicrobial peptides identified in various human tissues and secretions. This section will focus on antimicrobial peptides identified in resident skin cells, including human β-defensins (HBD-1, HBD-2, HBD-3), cathelicidin (LL-37), psoriasin, and RNase 7, which have all been demonstrated to be produced by keratinocytes, and dermcidin, which is secreted in human sweat. In addition, there are numerous other antimicrobial peptides that are produced by cells that infiltrate the skin and may participate in cutaneous innate immune responses.5
β-Defensins are cysteine-rich cationic low-molecular-weight antimicrobial peptides. The first human β-defensin, HBD-1, is constitutively expressed in the epidermis and is not transcriptionally regulated by inflammatory agents. HBD-1 has antimicrobial activity against Gram-negative bacteria and appears to play a role in keratinocyte differentiation. A second human β-defensin, HBD-2, was discovered in extracts of lesions from psoriasis patients.6 Unlike HBD-1 expression, HBD-2 expression is inducible by components of microbes, including Pseudomonas aeruginosa, Staphylococcus aureus, and Candida albicans.6 Not only can components of microbes stimulate expression of HBD-2, but proinflammatory cytokines such as tumor necrosis factor-α (TNF-α) and interleukin 1 (IL-1) can also induce HBD-2 transcription in keratinocytes.6 When tested for antimicrobial activity, HBD-2 was effective against Gram-negative bacteria such as Escherichia coli and P. aeruginosa and has a weak bacteriostatic effect against Gram-positive bacteria such as S. aureus.6 HBD-3 is another β-defensin that was first isolated from extracts of lesions from psoriasis patients.7 Contact with TNF-α and with bacteria was found to induce HBD-3 messenger RNA expression in keratinocytes. In addition, HBD-3 demonstrated potent bactericidal activity against S. aureus and vancomycin-resistant Enterococcus faecium. Therefore, HBD-3 is among the first human β-defensins in skin to demonstrate effective antimicrobial activity against Gram-positive bacteria. The localization of human β-defensins to the outer layer of the skin and the fact the β-defensins have antimicrobial activity against a variety of microbes suggest that human β-defensins are an essential part of cutaneous innate immunity. Furthermore, evidence indicating that human β-defensins attract DCs and memory T cells via CC chemokine receptor 6 (CCR6)8 provides a link between the innate and the adaptive immunity in skin.
Cathelicidins are cationic peptides with a structurally variable antimicrobial domain at the C-terminus. Whereas in mammals like pigs or cattle a variety of cathelicidin genes exists, men (and mice) possess only one gene. The human precursor protein hCAP18 (human cathelicidin antimicrobial protein 18) is produced by skin cells, including keratinocytes, mast cells, neutrophils, and ductal cells of eccrine glands. Neutrophil proteases (i.e., proteinase 3) process hCAP18 into the effector molecule LL-37 (named LL-37 for the 37-amino acid active antimicrobial peptide liberated from the C-terminus of the protein), which plays an important role in cutaneous host defense because of its pronounced antibacterial,9,10 antifungal,11 and antiviral12,13 activities. LL-37 further contributes to innate immunity by attracting mast cells and neutrophils via formyl peptide receptor-like 1 and by inducing mediator release from the latter cells via a G protein-dependent, immunoglobulin (Ig) E-independent mechanism.14 It has now been shown that LL-37 is secreted into human sweat, where it is cleaved by a serine protease-dependent mechanism into its peptides RK-31 or KS-30. Interestingly, these components display an even more potent antimicrobial activity than intact LL-37.15 One of the most important inducers of LL-37 expression is vitamin D, which can be triggered by Toll-like receptor (TLR) activation of the vitamin D receptor and vitamin D-1-hydroxylase genes, leading to enhanced antimicrobial killing.16,17
In atopic dermatitis (see Chapter 14), LL-37 is downregulated, probably due to the effect of the T2 cytokines IL-4 and IL-13, which renders atopic skin more susceptible to skin infections with, for example, S. aureus, vaccinia virus (eczema vaccinatum), or herpes simplex virus (HSV) (eczema herpeticum).10,12,13 Furthermore, patients with rosacea have been found to possess high levels of aberrantly processed forms of cathelicidin peptides (due to posttranslational processing by stratum corneum tryptic enzyme), which contributes to the increased inflammation in the skin.18 Cathelicidin can also form complexes with self-DNA, which promotes activation of TLR9 on plasmacytoid dendritic cells in the dermis, resulting in enhanced cutaneous inflammation that contributes to psoriasis pathogenesis.19
Another important human antimicrobial peptide has now been identified, psoriasin (S100A7),20 which elicits its antimicrobial effect by permeabilization of bacterial membranes.21 It is secreted predominantly by keratinocytes and plays a major role in killing the common gut bacterium E. coli. In fact, in vivo treatment of human skin with antipsoriasin antibodies results in the massive growth of E. coli.20 Furthermore, expression of psoriasin by keratinocytes has been shown to occur via TLR5 stimulation by E. coli flagellin.22 In addition to antimicrobial activity, psoriasin also functions as a chemoattractant for CD4 cells and neutrophils.23
RNase 7 was originally isolated from the stratum corneum from healthy human skin.24 RNase 7 has potent ribonuclease activity but also broad-spectrum antimicrobial activity against S. aureus, P. acnes, P. aeruginosa, E. coli, and C. albicans. RNase 7 production can be induced in cultured human keratinocytes by IL-1β, IFN-γ, and bacterial challenge. Interestingly, high expression of RNase 7 in human skin confers protection against S. aureus cutaneous infection.25
Dermcidin is an antimicrobial peptide that is expressed by human sweat glands.26 Dermcidin goes through postsecretory proteolytic processing in sweat that gives rise to anionic and cationic dermcidin peptides that are secreted onto the skin surface. These dermcidin peptides have broad antimicrobial activity against S. aureus, E. coli, E. faecalis, and C. albicans. Although the mechanism of action of dermcidin activity is unknown, it does not involve pore formation like other antimicrobial peptides.27
![]() Other secreted protein mediators that can be synthesized and released from keratinocytes and that may play a role in host defense are the complement components C3 and factor B. Keratinocytes are among the cells that synthesize eicosanoids, an ensemble of lipid mediators regulating inflammatory and immunologic reactions. They can produce and release the cyclooxygenase product prostaglandin E2, which has both proinflammatory and immunosuppressive properties and, when acting on DCs, promotes the development of IL-4-dominated type 2 T-cell responses.28 Other keratinocyte-derived eicosanoids include the neutrophil chemoattractant leukotriene B4, the proinflammatory 12-lipoxygenase product 12(s)-hydroxyeicosatetraenoic acid, and 15-hydroxyeicosatetraenoic acid, an anti-inflammatory and immunosuppressive metabolite of the 15-lipoxygenase pathway.
Other secreted protein mediators that can be synthesized and released from keratinocytes and that may play a role in host defense are the complement components C3 and factor B. Keratinocytes are among the cells that synthesize eicosanoids, an ensemble of lipid mediators regulating inflammatory and immunologic reactions. They can produce and release the cyclooxygenase product prostaglandin E2, which has both proinflammatory and immunosuppressive properties and, when acting on DCs, promotes the development of IL-4-dominated type 2 T-cell responses.28 Other keratinocyte-derived eicosanoids include the neutrophil chemoattractant leukotriene B4, the proinflammatory 12-lipoxygenase product 12(s)-hydroxyeicosatetraenoic acid, and 15-hydroxyeicosatetraenoic acid, an anti-inflammatory and immunosuppressive metabolite of the 15-lipoxygenase pathway.
![]() Another group of biologic response modifiers originating in keratinocytes and other epidermal cells is free radical molecules, now generally referred to as reactive oxygen species. These include the superoxide radical (O2−), hydrogen peroxide (H2O2), the hydroxyl radical (OH·), nitric oxide (NO), and others. These radicals are generally viewed as dangerously reactive entities threatening the integrity of many tissues. The skin is particularly at risk because it is exposed to oxygen from both inside and outside and because of the activation of oxygen by light (see Chapters 88 and 89). Free radicals probably contribute to solar damage and photoaging of the skin. However, certain reactive oxygen species have potent inflammation-inducing properties (e.g., free oxygen radicals) as well as immunomodulatory properties (e.g., NO), and thus provide an important host defense mechanism against microbial invasion. For discussion of these molecules, the reader is referred to the review by Bickers and Athar.29
Another group of biologic response modifiers originating in keratinocytes and other epidermal cells is free radical molecules, now generally referred to as reactive oxygen species. These include the superoxide radical (O2−), hydrogen peroxide (H2O2), the hydroxyl radical (OH·), nitric oxide (NO), and others. These radicals are generally viewed as dangerously reactive entities threatening the integrity of many tissues. The skin is particularly at risk because it is exposed to oxygen from both inside and outside and because of the activation of oxygen by light (see Chapters 88 and 89). Free radicals probably contribute to solar damage and photoaging of the skin. However, certain reactive oxygen species have potent inflammation-inducing properties (e.g., free oxygen radicals) as well as immunomodulatory properties (e.g., NO), and thus provide an important host defense mechanism against microbial invasion. For discussion of these molecules, the reader is referred to the review by Bickers and Athar.29
How do the cells of the innate immune system recognize foreign pathogens? One way that pathogens can be recognized and destroyed by the innate immune system is via receptors on phagocytic cells. Unlike adaptive immunity, the innate immune response relies on a relatively small set of germ line-encoded receptors that recognize conserved molecular patterns that are shared by a large group of pathogens. These are usually molecular structures required for survival of the microbes and therefore are not subject to selective pressure. In addition, pathogen-associated molecular patterns are specific to microbes and are not expressed in the host system. Therefore, the innate immune system has mastered a clever way to distinguish between self and nonself and relays this message to the adaptive immune system.
Of key importance was the discovery of the Toll-like receptors (TLRs), named after the Drosophila Toll gene whose protein product, Toll, participates in innate immunity and in dorsoventral development in the fruit fly.30,31 The importance of Toll signaling in mammalian cells was confirmed by the demonstration that the transmembrane leucine-rich protein TLR4 is involved in lipopolysaccharide (LPS) recognition.32
In addition to TLRs, there exist a variety of other molecules that sense the presence of pathogens. These include the NOD proteins (see below), triggering receptors expressed on myeloid cell (TREM) proteins,33 the family of Siglec molecules,34 and a group of C-type lectin receptors.35 The latter are prominently expressed on antigen-presenting cells (APCs) as, for instance, dectin-1 and DC-SIGN [DC-specific intercellular adhesion molecule 3 (ICAM-3) grabbing nonintegrin], which is actually expressed on tissue macrophages.36 They are able to mediate efficient binding of microorganisms; facilitate phagocytosis; and induce activation of signaling pathways that result in antimicrobial activity.
Members of the TREM protein family function as amplifiers of innate responses. Extreme examples of the consequences of microbe activation of TREM proteins are life-threatening septicemia and the deadly hemorrhagic fevers caused by Marburg and Ebola virus infection.37
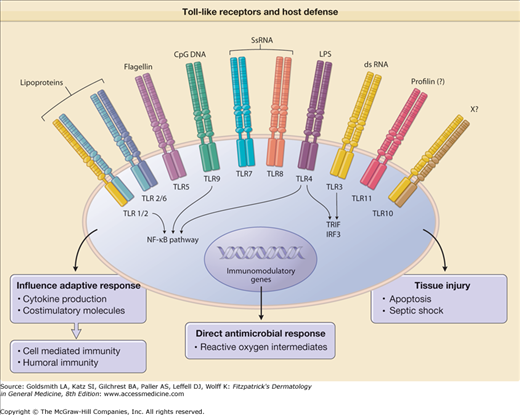
There is now substantial evidence to support a role for mammalian TLRs in innate immunity (Fig. 10-3). First, TLRs recognize pathogen-associated molecular patterns present in a variety of bacteria, fungi, and viruses. Second, TLRs are expressed at sites that are exposed to microbial threats. Third, the activation of TLRs induces signaling pathways that, on the one hand, stimulate the production of antimicrobial effector molecules, and, on the other, promote the expression of costimulatory molecules and the release of cytokines and, as a result, the augmentation of the adaptive response. Fourth, TLRs directly activate host defense mechanisms that then combat the foreign invader.
Figure 10-3
Toll-like receptors (TLRs) mediate innate immune response in host defense. Activation of TLRs by specific ligands induces (1) cytokine release and costimulatory molecules that instruct the type of adaptive immune response; (2) direct antimicrobial response; and (3) tissue injury. CpG DNA = immunostimulatory cytosine- and guanine-rich sequences of DNA; dsRNA = double-stranded RNA; LPS = lipopolysaccharide; NF-κB = nuclear factor κB; ssRNA = single-stranded RNA; X = ligand unknown.
Experiments performed in the Modlin laboratory39 and others40 led to the exciting finding that microbial lipoproteins trigger host responses via TLR2, requiring the acyl functions for activity. Subsequently, triacylated lipoproteins were found to activate TLR2/1 heterodimers,41 whereas diacylated lipoproteins were found to activate TLR2/6 heterodimers.42 For recognition of bacteria, the TLR system is redundant: TLR9 is activated by unmethylated DNA sequences (CpG dinucleotides) found in bacterial DNA43 and TLR5 activated by bacterial flagellin.44 Specific TLRs are involved in viral recognition: TLR3 is activated by viral derived double-stranded RNA45 and TLR7 and TLR8 by virus-derived single-stranded RNA.46 The finding that different TLRs have distinct patterns of expression, particularly on monocytes, macrophages, dendritic cells, B cells, endothelia, and epithelia, suggests that each TLR could trigger a specific host response. Furthermore, TLRs are expressed in specific subcellular compartments: TLR7, 8, and 9 are located in endosomes, where they encounter microbial pathogens in the endocytic pathway. The other TLRs are expressed on the cell surface and detect microbial ligands in the extracellular environment.
The expression of TLRs on cells of the monocyte/macrophage lineage is consistent with the role of TLRs in modulating inflammatory responses via cytokine release. Because these cells migrate into sites that interface with the environment—lung, skin, and gut—the location of TLR-expressing cells would situate them to defend against invading microbes. TLR expression by adipocytes, intestinal epithelial cells, and dermal endothelial cells supports the notion that TLRs serve a sentinel role with regard to invading microorganisms. The regulation of TLR expression is critical to their role in host defense, yet few factors have been identified that modulate this process. IL-4 acts to downregulate TLR expression,47 which suggests that T helper 2 (T2) adaptive immune responses might inhibit TLR activation.
TLR activation of a variety of cell types has been shown to trigger release of both proinflammatory and immunomodulatory cytokines.48–52 TLR activation of monocytes and DC induces IL-12 and IL-18, required for generation of a Th1 response, and IL-1β, IL-6, IL-23, involved in the generation of a Th17 response, as well as the anti-inflammatory IL-10.53–56 The relative induction of specific cytokine patterns determines the type of adaptive T-cell response (see Chapter 11).
TLRs can regulate phagocytosis either through enhancing endosomal fusion with the lysosomal compartment57 or through induction of a phagocytic gene program including multiple scavenger receptors.58 Activation of TLRs on monocytes leads to the induction of IL-15 and IL-15R, triggering differentiation into CD209+ MΦ36 with microbicidal activity.59 Activation of TLRs on monocytes also induces GM-CSF and GM-CSFR, triggering differentiation into immature DC with the capacity to release cytokines and efficiently present antigen to T cells.36 In addition, activation of TLRs on immature DC leads to further maturation with enhanced T-cell stimulatory capacity.60
In Drosophila, Toll is critical for host defense. The susceptibility of mice with spontaneous mutations in TLRs to bacterial infection indicates that mammalian TLRs play a similar role. Activation of TLR2 by microbial lipoproteins induces activation of the inducible nitric oxide (NO) synthase (NOS-II or iNOS) promoter,39 which leads to the production of NO, a known antimicrobial agent. There is strong evidence that TLR2 activation leads to killing of intracellular Mycobacterium tuberculosis in both mouse and human macrophages.54 In mouse macrophages, bacterial lipoprotein activation of TLR2 leads to a NO-dependent killing of intracellular tubercle bacilli. In human monocytes and alveolar macrophages, bacterial lipoproteins similarly activate TLR2 to kill intracellular M. tuberculosis; however, this occurs by an antimicrobial pathway that is NO-independent. Instead, a key antimicrobial mechanism for TLR-activated human monocytes involves induction of the 25-hydroxyvitamin D3-1α-hydroxylase (CYP27b1), which converts the 25D into the active 1,25D form, upregulation and activation of the vitamin D receptor (VDR), and downstream induction of the antimicrobial peptide cathelicidin.16,59,61–63 The ability of TLR2/1 activation to upregulate expression of CYP27b1 and the VDR is IL-15 dependent.36 Simultaneous triggering of IL-1β activity and activation of the VDR induces HBD-2, also required for antimicrobial activity.
Activation of TLRs 3, 4, 7, 8, and 9 leads to induction of antiviral activity, dependent on type I IFN secretion and involving specific signaling pathways.64 Two TLR-mediated pathways have been identified: type I IFN production occurs through a MyD88-independent pathway in response to TLR3 and TLR4 activation,65 and, following stimulation with agonists of TLRs 7, 8, and 9, through a MyD88-dependent pathway.66
The activation of TLRs can also be detrimental, leading to tissue injury. The administration of LPS to mice can result in manifestations of septic shock, which is dependent on TLR4.32 Evidence suggests that TLR2 activation by Propionibacterium acnes induces inflammatory responses in acne vulgaris, which lead to tissue injury.67 Aliprantis et al demonstrated that microbial lipoproteins induce features of apoptosis via TLR2.40 Thus, microbial lipoproteins have the ability to elicit both TLR-dependent activation of host defense and tissue pathology. This dual signaling pathway is similar to TNF receptor and CD40 signaling, which leads to both nuclear factor-κB activation and apoptosis.68,69 In this manner, it is possible for the immune system to use the same molecules to activate host defense mechanisms and then, by apoptosis, to downregulate the response from causing tissue injury. Activation of TLR can lead to the inhibition of the major histocompatibility complex (MHC) class II antigen presentation pathway, which can downregulate immune responses leading to tissue injury but may also contribute to immunosuppression.70 Finally, Toll activation has been implicated in bone destruction.52
The critical biologic role of TLRs in human host defense can be deduced from the finding that TLR4 mutations are associated with LPS hyporesponsiveness in humans.71 By inference, one can anticipate that humans with genetic alterations in TLR may have increased susceptibility to certain microbial infections. Furthermore, it should be possible to exploit the pathway of TLR activation as a means to endorse immune responses in vaccines and treatments for infectious diseases as well as to abrogate responses detrimental to the host.
![]() In contrast to TLRs, nucleotide-binding oligomerization domain proteins (NOD1 and NOD2) are found free in the cytosol and detect breakdown products of peptidoglycan.72,73 NOD1 recognizes breakdown products of Gram-negative peptidoglycan whereas NOD2 recognizes muramyl dipeptide (MDP), which is a breakdown product of peptidoglycan from both Gram-positive and Gram-negative bacteria. After ligand detection, NODs activate a signaling pathway that results in NF-kB activation, through the adapter molecule RIP2, and transcription of host genes involved in innate and acquired immune responses. In addition, NOD2 can also activate the inflammasome leading to the proteolytic cleavage and activation of IL-1β.74,75 NOD1 and NOD2 are thought to be primarily important in recognizing intracellular pathogens. However, extracellular bacteria can invade the cytoplasm of cells and lead to activation of NOD2. This has been demonstrated in the case of S. aureus skin infection.76 Further studies are needed to determine the role of NOD1 and NOD2 against other skin pathogens. Interestingly, mutations in NOD2 are associated with Crohn’s disease, sarcoidosis, and Blau’s syndrome, which is a disease consisting of early-onset granulomatous inflammation (arthritis, uveitis, skin), visceral involvement, and camptodactyly.77–79 In addition, polymorphisms in NOD2 and the NOD2 signaling pathway have been associated with leprosy, suggesting that all these diseases may be mechanistically linked.80 Furthermore, NOD1 polymorphisms have been associated with atopic dermatitis and asthma.81
In contrast to TLRs, nucleotide-binding oligomerization domain proteins (NOD1 and NOD2) are found free in the cytosol and detect breakdown products of peptidoglycan.72,73 NOD1 recognizes breakdown products of Gram-negative peptidoglycan whereas NOD2 recognizes muramyl dipeptide (MDP), which is a breakdown product of peptidoglycan from both Gram-positive and Gram-negative bacteria. After ligand detection, NODs activate a signaling pathway that results in NF-kB activation, through the adapter molecule RIP2, and transcription of host genes involved in innate and acquired immune responses. In addition, NOD2 can also activate the inflammasome leading to the proteolytic cleavage and activation of IL-1β.74,75 NOD1 and NOD2 are thought to be primarily important in recognizing intracellular pathogens. However, extracellular bacteria can invade the cytoplasm of cells and lead to activation of NOD2. This has been demonstrated in the case of S. aureus skin infection.76 Further studies are needed to determine the role of NOD1 and NOD2 against other skin pathogens. Interestingly, mutations in NOD2 are associated with Crohn’s disease, sarcoidosis, and Blau’s syndrome, which is a disease consisting of early-onset granulomatous inflammation (arthritis, uveitis, skin), visceral involvement, and camptodactyly.77–79 In addition, polymorphisms in NOD2 and the NOD2 signaling pathway have been associated with leprosy, suggesting that all these diseases may be mechanistically linked.80 Furthermore, NOD1 polymorphisms have been associated with atopic dermatitis and asthma.81
Two key cells of the innate immune system are characterized by their phagocytic function: macrophages and PMNs. These cells have the capacity to take up pathogens, recognize them, and destroy them. Some of the functions of these cells are regulated via TLRs and complement receptors as outlined earlier.
PMNs are normally not present in skin; however, during inflammatory processes, these cells migrate to the site of infection and inflammation, where they are the earliest phagocytic cells to be recruited. These cells have receptors that recognize pathogens directly (see Pattern Recognition Receptors), and due to their expression of FcγRIII/CD16 and C3bR/CD35, can phagocytose microbes coated with antibody and with the complement component C3b. As a consequence, granules (containing myeloperoxidase, elastase, lactoferrin, collagenase, and other enzymes) are released, and microbicidal superoxide radicals (O2−) are generated (see Chapter 30).
Activation of phagocytes by pathogens induces several important effector mechanisms, for example, triggering of cytokine production. A number of important cytokines are secreted by macrophages in response to microbes, including IL-1, IL-6, TNF-α, IL-8, IL-12, and IL-10 (see also Chapter 11).
Another important defense mechanism triggered in phagocytes in response to pathogens is the induction of direct antimicrobial responses. Phagocytic cells such as PMNs and macrophages recognize pathogens, engulf them, and induce antimicrobial effector mechanisms to kill the pathogens. The induction and/or release of toxic oxygen radicals, lysosomal enzymes, and antimicrobial peptides leads to direct killing of microbial organisms.4 Similarly, activation of TLRs on macrophages induces these various antimicrobial pathways as already discussed above.
Cytokines of the adaptive T-cell response influence macrophage differentiation: IFN-γ treatment results in “classically activated” macrophages, with antimicrobial activity, whereas in contrast IL-4 or IL-13 triggers differentiation into “alternatively activated” macrophages, which contribute to humoral and antiparasite immunity.82,83 Cytokines produced by the innate immune response also induce distinct macrophage differentiation programs.84 IL-10 induces the phagocytic program in macrophages, leading to the uptake of lipids and bacteria. In contrast, IL-15 induces a macrophage antimicrobial program. These data establish that the innate immune response, by selectively inducing IL-10 versus IL-15, differentially programs macrophages for phagocytosis versus antimicrobial responses that largely determines the outcome of infection.
Phagocytic cells of the innate immune system can also be activated by cells of the adaptive immune system. CD40 is a 50-κDa glycoprotein present on the surface of B cells, monocytes, DCs, and endothelial cells. The ligand for CD40 is CD40L, a type II membrane protein of 33 kDa, preferentially expressed on activated CD4+ T cells and mast cells. CD40−CD40 ligand interaction plays a crucial role in the development of effector functions. CD4+ T cells activate macrophages and monocytes to produce TNF-α, IL-1, IL-12, interferon-γ (IFN-γ), and NO via CD40–CD40L interaction. CD40L has also been shown to rescue circulating monocytes from apoptotic death, thus prolonging their survival at the site of inflammation. In addition, CD40–CD40L interaction during T-cell activation by APCs results in IL-12 production. Therefore, it can be concluded that CD40–CD40L interactions between T cells and macrophages play a role in maintenance of T1-type cellular responses and mediation of inflammatory responses. Other studies have established a role for CD40–CD40L interactions in B-cell activation, differentiation, and Ig class switching.85 In addition, CD40–CD40L interaction leads to upregulation of B7.1 (CD80) and B7.2 (CD86) on B cells. This costimulatory activity induced on B cells then acts to amplify the response of T cells. These mechanisms underscore the importance of the interplay between the innate and the adaptive immune system in generating an effective host response.
![]() (See Chapter 31). Eosinophils are a distinct class of bone marrow-derived granulocytes that normally constitute only a small fraction of peripheral blood leukocytes and occur in even smaller numbers in peripheral tissues. The cytokines granulocyte–macrophage colony-stimulating factor (GM-CSF), IL-3 and, most importantly, IL-5 are critical for their development and maturation.
(See Chapter 31). Eosinophils are a distinct class of bone marrow-derived granulocytes that normally constitute only a small fraction of peripheral blood leukocytes and occur in even smaller numbers in peripheral tissues. The cytokines granulocyte–macrophage colony-stimulating factor (GM-CSF), IL-3 and, most importantly, IL-5 are critical for their development and maturation.
NK cells appear as large granular lymphocytes. In humans, the vast majority of these cells exhibit the CD3−, CD56+, CD16+, CD94+, and CD161+ phenotype. Their function is to survey the body looking for altered cells, be they transformed or infected with viruses (e.g., cytomegalovirus), bacteria (e.g., Listeria monocytogenes), or parasites (e.g., Toxoplasma gondii). These pathogens are then killed directly via perforin/granzyme- or Fas/Fas ligand (FasL)-dependent mechanisms or indirectly via the secretion of cytokines (e.g., IFN-γ).
All nucleated cells express the MHC class I molecules. NK cells have receptors, termed killer inhibitory receptors, which recognize the self-MHC class I molecules. This recognition results in the delivery of a negative signal to the NK cell that paralyzes it. If a nucleated cell loses expression of its MHC class I molecules, however, as often happens after malignant transformation or virus infection, the NK cell, on encountering it, will become activated and kill it.
In addition, NK cells have activating receptors that bind MHC-like ligands on target cells. One such receptor is NKGD2, which binds to the human nonclassic MHC class I chain-related A and B molecules, MICA and MICB.87 MICA and MICB are not expressed in substantial amounts on normal tissues, but are overexpressed on carcinomas.88 NK cells are able to kill MICA/MICB-bearing tumors, which suggests a role for NKGD2 in immune surveillance.
Another cell type that, at least in mice, could serve a similar function is the IFN-producing killer DC, which shares several features with DCs and NK cells.89,90 Their human equivalent has yet to be identified.
Once thought to only play a role in maintaining the physical barrier of the skin, keratinocytes, the predominant cells in the epidermis, can participate in innate immunity by mounting an immune and/or inflammatory response through secretion of cytokines and chemokines, arachidonic acid metabolites, complement components, and antimicrobial peptides.
Keratinocytes of unperturbed skin produce only a few of these mediators, such as the cytokines IL-1, IL-7, and transforming growth factor-β (TGF-β), constitutively. Resident keratinocytes contain large quantities of preformed and biologically active IL-1α as well as immature IL-1β in their cytoplasm.91 The likely in vivo role of this stored intracellular IL-1 is that of an immediate initiator of inflammatory and repair processes after epidermal injury. IL-7 is an important lymphocyte growth factor that may have a role in the survival and proliferation of the T lymphocytes of human skin. Some evidence exists for the IL-7-driven propagation of lymphoma cells in Sézary syndrome.
TGF-β, in addition to its growth-regulating effects on keratinocytes and fibroblasts, modulates the inflammatory as well as the immune response92 and is important for LC development (see in Langerhans Cells).93 On delivery of certain noxious, or at least potentially hazardous, stimuli (e.g., hypoxia, trauma, nonionizing radiation, haptens, or other rapidly reactive chemicals like poison ivy catechols, silica, LPS, and microbial toxins), the production and/or release of many cytokines is often dramatically enhanced. The biologic consequences of this event are manifold and include the initiation of inflammation (IL-1, TNF-α, IL-6, members of the chemokine family), the modulation of LC phenotype and function (IL-1, GM-CSF, TNF-α, IL-10, IL-15), T-cell activation (IL-15, IL-18),94,95 T-cell inhibition (IL-10, TGF-β),96 and skewing of the lymphocytic response in either the type 1 (IL-12, IL-18),97 type 2 (thymic stromal lymphopoietin),98 or Th17 (IL-23) direction.99 In some cases, keratinocytes may also play a role in amplifying inflammatory signals in the epidermis originating from numerically minor epidermal cell subsets. One prominent example is the induction of proinflammatory cytokines such as TNF-α in keratinocytes by LC-derived IL-1β in the initiation phase of allergic contact dermatitis.100 In the presence of a robust stimulus, keratinocyte-derived cytokines may be released into the circulation in quantities that cause systemic effects. During a severe sunburn reaction, for example, serum levels of IL-1, IL-6, and TNF-α are clearly elevated and probably responsible for the systemic manifestations of this reaction, such as fever, leukocytosis, and the production of acute-phase proteins.101 There is also evidence that the ultraviolet (UV) radiation-inducible cytokines IL-6 and IL-10 can induce the production of autoantibodies and thus be involved in the exacerbation of autoimmune diseases such as lupus erythematosus. The fact that secreted products of keratinocytes can reach the circulation could conceivably also be used for therapeutic purposes. The demonstration by Fenjves et al102 that grafting of apolipoprotein E gene-transfected human keratinocytes onto mice results in the detection of apolipoprotein E in the circulation of the mouse supports the feasibility of such an approach.
Some of the innate functions of keratinocytes can be elicited by TLR activation, since keratinocytes express TLRs 1–6 and 9. Thus, by sensing microbial pathogens via TLRs, keratinocytes may act as first-responders in cutaneous innate immunity. Activation of TLRs leads to keratinocyte production of proinflammatory cytokines (including TNF-α and IL-8), antimicrobial peptides (HBD-2 and HBD-3), and reactive oxygen mediators (iNOS).103–105 Activation of TLR3 and TLR9 on keratinocytes induces production of type I interferon (IFN-α/β), which may be important in promoting antiviral immune responses.105 Lastly, these TLR-mediated responses can be enhanced via danger signals such as toxins, irritants, UV light, purines generated during an infection (P2×7 receptor activation), and activation of other pattern-recognition receptors (NOD1 and NOD2), which all promote inflammasome-mediated activation of caspase-1 that results in cleavage of pro-IL1β into its active form.106
Another important function of keratinocytes is the production/secretion of factors governing the influx and efflux of leukocytes into and out of the skin. Two good examples are the chemokines thymus and activation-regulated chemokine (TARC; CC chemokine ligand 17, or CCL17) and cutaneous T cell-attracting chemokine (CTACK)/CCL27 and their corresponding receptors CCR4 and CCR10, selectively expressed on skin-homing T lymphocytes. Blocking of both chemokines drastically inhibits the migration of T cells to the skin in a murine model of contact hypersensitivity (CHS).107 KC-derived macrophage inflammatory protein 3α (MIP-3α)/CCL20 also plays an important role in leukocyte recruitment to the epidermis. Its secretion is triggered or enhanced by IL-17 and its counterreceptor CCR6 is present on LC precursors and certain T cells.108–110 The T17 cytokines, IL-17, IL-21, and IL-22 also modulate other keratinocyte innate immune functions. For example, IL-17 and IL-22 promote keratinocyte production of antimicrobial peptides, including HBD-2, cathelicidin, and psoriasin.111,112 In addition, IL-21 and IL-22 induce keratinocyte proliferation, leading to epidermal hyperplasia and acanthosis as seen in psoriasis.113,114
The demonstration of cytokine receptors on and cytokine responsiveness of keratinocytes established that the functional properties of these cells can be subject to regulation by cells of the immune system. As a consequence, keratinocytes express, or are induced to express, immunologically relevant surface moieties that can be targeted by leukocytes for stimulatory or inhibitory signal transduction.
In addition to cytokines, keratinocytes secrete other factors such as neuropeptides, eicosanoids, and reactive oxygen species. These mediators have potent inflammatory and immunomodulatory properties and play an important role in the pathogenesis of cutaneous inflammatory and infectious diseases as well as in aging.
Keratinocytes synthesize complement and related receptors including the C3b receptor [complement receptor 1 (CR1), CD35], the Epstein-Barr virus receptor CR2 (C3d receptor, CD21), the C5a receptor (CD88), the membrane cofactor protein (CD46), the decay-accelerating factor (CD55), and complement protectin (CD59). CD59 may protect keratinocytes from attack by complement. Its engagement by CD2 stimulates the secretion of proinflammatory cytokines from keratinocytes. Membrane cofactor (CD46) is reported to be a receptor for M protein of group A Streptococci and for measles virus.115 Its ligation induces proinflammatory cytokines in keratinocytes such as IL-1α, IL-6, and GM-CSF.
Adaptive Immune Response
The strength and the type of the innate response determines both the quantity and quality of an adaptive response initiated by dendritic APCs in the epidermis (LCs) and dermis (dermal DCs or DDCs) and executed by T lymphocytes and antibodies.
Three subsets of lymphocytes exist in the human immune system: B cells, T cells, and NK cells (see Section “Cells of the Innate Immune System”). The adaptive immune response is mediated by T and B lymphocytes. The unique role of these cells is the ability to recognize antigenic specificities in all their diversity. All lymphocytes derive from a common bone marrow stem cell. This finding has been exploited in various clinical settings, with attempts to restore the entire lymphocyte pool by bone marrow or stem cell transplantation.
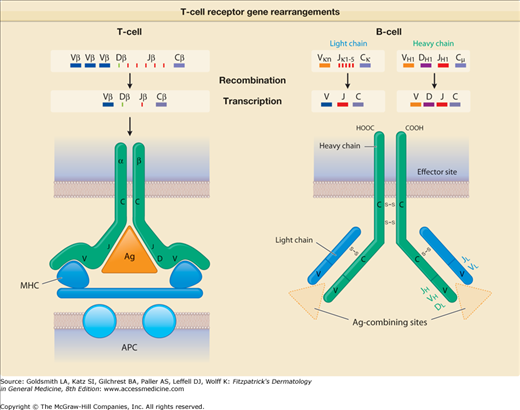
B cells mature in the fetal liver and adult bone marrow. They produce antibody-protein complexes that bind specifically to particular molecules defined as antigens. As a consequence of recombinatorial events in different Ig gene segments (V or variable; D or diversity; J or joining), each B cell produces a different antibody molecule (eFig. 10-3.1). Some of this antibody is present on the surface of the B cell, conferring the unique ability of that B cell to recognize a specific antigen. B cells then differentiate into plasma cells, the actual antibody-producing and -secreting cells. Plasma-cell secreted Ig comprise the dimer IgA, the monomers IgD, IgE, and IgG as well as the pentamer IgM that mediate humoral immune responses. In general, antibodies bind to microbial agents and neutralize them or facilitate uptake of the pathogen by phagocytes that destroy them. Briefly, IgA can be found in mucosal tissues, saliva, tears, or breast milk and prevents colonization by various pathogens. IgD functions mainly as an antigen receptor on B cells and, as recently discovered, activates mast cells and basophils to produce antimicrobial factors.116 IgE binds to allergens on mast cells and basophils and can thereby trigger histamine release and allergic reactions including anaphylaxis and urticaria. In addition, some evidence exists that it can protect against parasitic and helminthic infections. IgG provides the majority of antibody responses that contribute to the immune defense against extracellular pathogens. It is the only antibody that is capable of crossing the placenta in order to protect the fetus. Finally, IgM is available either surface-bound on B cells or as secreted form and eliminates microbes in the early stages of humoral immunity before there is sufficient IgG production. Antibodies are also responsible for mediating certain pathologic conditions in skin. In particular, antibodies against self-antigens (mostly IgG, but also IgA) lead to autoimmune disease, typified in the pathogenesis of pemphigus and bullous pemphigoid (see Chapter 37 for more details about B cells and antibody production).
eFigure 10-3.1
T-cell receptor (TCR) gene rearrangements. This diagram shows how diversity in TCRs and antibodies is generated by gene rearrangement. For the TCR, rearrangement of the β chain is shown, and for antibodies, that of immunoglobulin M heavy and light chains is depicted. The encoded antibody recognizes the nominal antigen per se, whereas the encoded TCR recognizes antigen in the context of an appropriate antigen-presenting molecule. Ag = antigen; APC = antigen-presenting cell; C = constant segment; D = diversity segment; J = joining segment; MHC = major histocompatibility complex; V = variable segment.
T cells mature in the thymus, where they are selected to live or to die. Those T cells that will have the capacity to recognize foreign antigens are positively selected and can enter the circulation. Those T cells that react to self are negatively selected and destroyed. T cells have the unique ability to direct other cells of the immune system. They do this, in part, by releasing cytokines. For example, T cells contribute to cell-mediated immunity (CMI), required to eliminate intracellular pathogens, by releasing cytokines that activate macrophages and other T cells. T cells release cytokines that activate NK cells and permit the growth, differentiation, and activation of B cells.
T cells can be classified and subdivided in different ways: (1) on the basis of the T cell receptor; (2) on the basis of the accessory molecules CD4 and CD8; (3) on the basis of their virginity, i.e., their activation status (naive, memory, effector T cells); and (4) on the basis of their functional role in the immune response, which is often linked to the cytokine secretion property of the respective cell population. We have used the abbreviations Th1 and Th2 to distinguish CD4+ helper T cell subtypes but, as discussed below, many of the functional attributes, including cytokine production, of Th cells are not as clearly defined as previously thought and some cytokine profiles are also attributable to CD8+ cytotoxic T cells (Tc) (see Section “Functionality”).
The T-cell antigen receptor (TCR) is a complex of molecules consisting of an antigen-binding heterodimer (α/β or γ/δ chains) that is noncovalently linked with five CD3 subunits [(1) γ, (2) δ, (3) ε, (4) ζ, or (5) η). The TCR chains have amino acid sequence homology with structural similarities to Ig heavy and light chains. The genes encoding TCR molecules are encoded as clusters of gene segments (V, J, D, C, or constant) that rearrange during T-cell maturation (eFig. 10-3.1). Together with the addition of nucleotides at the junction of rearranged gene segments, this recombinatorial process, which involves the enzymes recombinase activating gene 1 and 2, results in a heterogeneity and diversity of the antigen recognition unit that is broad enough to allow for a successful host defense. TCR α/β or TCR γ/δ molecules must be paired with CD3 molecules to be inserted into the T-cell surface membrane117 (see Fig. 10-4). The TCR chains form the actual antigen-binding unit, whereas the CD3 complex mediates signal transduction, which results in either productive activation or nonproductive silencing of the T lymphocyte. Most T cells express α/β TCRs, which typically bind antigenic peptides presented by MHC molecules. Immunity provided by 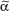 /β T cells includes Th1, Th2, Th17 and T reg responses (see Section “Functionality”). In contrast, only a small subset of T cells express γ/δ TCRs. These T cells have the capacity to directly bind pathogen-derived glycoproteins or nonclassical MHC molecules. It has been shown that γ/δ T cells in men and mice predominantly display a tissue-associated TCR repertoire as well as a memory phenotype, both probably due to chronical stimulation by nonpeptide antigens within the tissue. Importantly, they act early during immune response and are therefore termed “innate-like effectors.” Previous studies conducted in mice infected with Listeria monocytogenes or Nippostrongylus brasiliensis revealed that
/β T cells includes Th1, Th2, Th17 and T reg responses (see Section “Functionality”). In contrast, only a small subset of T cells express γ/δ TCRs. These T cells have the capacity to directly bind pathogen-derived glycoproteins or nonclassical MHC molecules. It has been shown that γ/δ T cells in men and mice predominantly display a tissue-associated TCR repertoire as well as a memory phenotype, both probably due to chronical stimulation by nonpeptide antigens within the tissue. Importantly, they act early during immune response and are therefore termed “innate-like effectors.” Previous studies conducted in mice infected with Listeria monocytogenes or Nippostrongylus brasiliensis revealed that 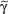 /δ T cells discriminate early between these pathogens and react by IFN-γ versus IL-4 production, skewing
/δ T cells discriminate early between these pathogens and react by IFN-γ versus IL-4 production, skewing 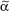 /β T-cell responses in a Th1 or Th2 direction, respectively.118 Meanwhile, growing evidence exists that human and murine
/β T-cell responses in a Th1 or Th2 direction, respectively.118 Meanwhile, growing evidence exists that human and murine 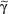 /δ T cells also have the capacity to produce IL-17 during bacterial or viral infections and thereby significantly contribute to the early innate immune defense.119–121
/δ T cells also have the capacity to produce IL-17 during bacterial or viral infections and thereby significantly contribute to the early innate immune defense.119–121
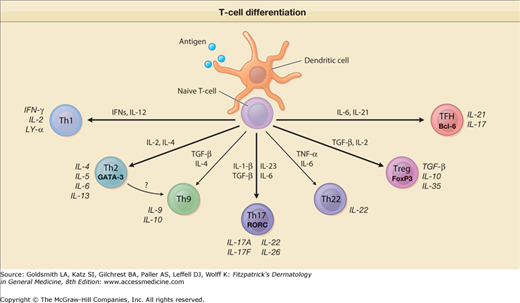
Figure 10-4
Schematic view of events governing and occurring in T-cell differentiation. Depending on the type and activation status of the antigen-presenting dendritic cells (DCs) and on the type and amounts of cytokines secreted by these and/or other cells, naive T cells will expand and differentiate into various directions, i.e., Th1 cells, Th2 cells, Th9 cells, Th17 cells, Th22 cells, T reg cells, and Tfh cells. They exhibit different types of transcription factors (e.g., T-bet, GATA-3, RORC, FoxP3, Bcl-6) and secrete different types of cytokines.
![]() During their maturation in the thymus thymocytes start to express the molecules that allow T cells to display their unique functional capacity, which is to specifically recognize antigen in an MHC-restricted fashion (see Section “General Principles of Antigen Presentation”). These are the TCR and the accessory molecules CD4 and CD8. The latter stabilize the interaction of the TCR with the MHC-linked peptide antigen. Whereas CD4 binds to MHC class II molecules, CD8 acts as an adhesive by binding to MHC class I molecules. Thymocyte development follows a strict selection process. First, lymphoid progenitor cells enter the thymus and develop into CD25+CD4−CD8− (double-negative, DN) thymocytes. Upon successful generation of functional TCR-b and pre-TCR-α receptors, further development to CD4+CD8+ (double-positive, DP) thymocytes with fully functional TCR-αβ chains is initiated. Following low-avidity TCR recognition of self-peptide/MHC molecules, DP thymocytes receive signals for survival and further differentiate into single positive (SP) thymocytes. These positively selected mature thymocytes constitute only 3%–5% of all thymocytes and are either CD4+CD8− MHC class II–restricted cells or CD8+CD4− MHC class I-restricted cells. Subsequently, they leave the thymus and migrate to the peripheral lymphoid tissues (lymph nodes, spleen, Peyer’s patches, etc.). On the contrary, thymocytes that show self-reactivity undergo apoptosis in order to avoid autoimmunity (negative selection), and DP thymocytes that do not receive TCR signals die due to neglect. This process is most active in early infancy and childhood but continues with decreasing output well into adult life.
During their maturation in the thymus thymocytes start to express the molecules that allow T cells to display their unique functional capacity, which is to specifically recognize antigen in an MHC-restricted fashion (see Section “General Principles of Antigen Presentation”). These are the TCR and the accessory molecules CD4 and CD8. The latter stabilize the interaction of the TCR with the MHC-linked peptide antigen. Whereas CD4 binds to MHC class II molecules, CD8 acts as an adhesive by binding to MHC class I molecules. Thymocyte development follows a strict selection process. First, lymphoid progenitor cells enter the thymus and develop into CD25+CD4−CD8− (double-negative, DN) thymocytes. Upon successful generation of functional TCR-b and pre-TCR-α receptors, further development to CD4+CD8+ (double-positive, DP) thymocytes with fully functional TCR-αβ chains is initiated. Following low-avidity TCR recognition of self-peptide/MHC molecules, DP thymocytes receive signals for survival and further differentiate into single positive (SP) thymocytes. These positively selected mature thymocytes constitute only 3%–5% of all thymocytes and are either CD4+CD8− MHC class II–restricted cells or CD8+CD4− MHC class I-restricted cells. Subsequently, they leave the thymus and migrate to the peripheral lymphoid tissues (lymph nodes, spleen, Peyer’s patches, etc.). On the contrary, thymocytes that show self-reactivity undergo apoptosis in order to avoid autoimmunity (negative selection), and DP thymocytes that do not receive TCR signals die due to neglect. This process is most active in early infancy and childhood but continues with decreasing output well into adult life.
The original observation that CD4+ T cells are critical for helping B cells to produce antibodies by triggering their differentiation into plasma cells in the humoral response coined the term “T helper cells” (Th cells). During the past years these lymphocytes have been characterized extensively. To our current knowledge, CD4+ T cells represent a heterogeneous cell population with diverse function depending on environmental requirements that play a central role in humoral and cell-mediated immunity. Effector CD4+ T cells protect against pathogens mainly by their production of Th1, Th2, or Th17 cytokines (i.e., IFN-γ, IL-4, IL-17) and influence immune responses through both “helper” and “effector” functions. In contrast, regulatory CD4+ T cells have the capacity to downregulate disproportionate effector responses to (self-) antigen (see Section “Functionality”).
In responding to an intracellular pathogen (e.g., a virus) the T cell must lyse the infected cell. To do so, it must be able to recognize and respond to antigenic peptides encoded by this pathogen and displayed on the cell surface. For this to occur, antigens arising in the cytosol are cleaved into small peptides by a complex of proteases, called the proteasome. The peptide fragments are then transported from the cytosol into the lumen of the endoplasmic reticulum, where they associate with MHC class I molecules. These peptide–class I complexes are exported to the Golgi apparatus and then to the cell surface (see Section “General Principles of Antigen Presentation”). The maturation of a CD8+ T cell to a killer T cell requires not only the display of the antigenic signal but also the delivery of helper signals from CD4+ T cells, for which the functional interaction between CD40 on the APC and CD40L on the CD8+ T cell can substitute.
![]() Two distinct subsets of cytotoxic T cells have been identified and can be differentiated by the mechanism by which they kill targets124; the end result being the induction of a programed cell death known as apoptosis.125,126 The first mechanism of cytotoxicity involves the interaction of two cell surface proteins, FasL (CD95L) on the T cells and Fas (CD95) on the target. Ligation of these molecules delivers a signal through Fas that induces the apoptosis cascade in the target. The second mechanism involves the release of cytoplasmic granules present in such T cells. These granules contain perforin, which induces a pore in the target, and granzymes, serine esterases that, when injected into cells, trigger the apoptotic pathway. Such granules also contain granulysin, a protein with a broad spectrum of antimicrobial activity against bacteria, fungi, and parasites.124,127 In this manner, cytotoxic T cells can directly kill microbial invaders. Besides contributing to host defense against infection and tumors, cytotoxic T cells can also contribute to tissue injury. For example, cytotoxic T cells exist which recognize self-antigens of melanocytes and thus may contribute to the pathogenesis of vitiligo.128
Two distinct subsets of cytotoxic T cells have been identified and can be differentiated by the mechanism by which they kill targets124; the end result being the induction of a programed cell death known as apoptosis.125,126 The first mechanism of cytotoxicity involves the interaction of two cell surface proteins, FasL (CD95L) on the T cells and Fas (CD95) on the target. Ligation of these molecules delivers a signal through Fas that induces the apoptosis cascade in the target. The second mechanism involves the release of cytoplasmic granules present in such T cells. These granules contain perforin, which induces a pore in the target, and granzymes, serine esterases that, when injected into cells, trigger the apoptotic pathway. Such granules also contain granulysin, a protein with a broad spectrum of antimicrobial activity against bacteria, fungi, and parasites.124,127 In this manner, cytotoxic T cells can directly kill microbial invaders. Besides contributing to host defense against infection and tumors, cytotoxic T cells can also contribute to tissue injury. For example, cytotoxic T cells exist which recognize self-antigens of melanocytes and thus may contribute to the pathogenesis of vitiligo.128
Double-negative (DN) T cells comprise only 1%–5% of the peripheral T-cell population of mice and men. DN T cells can be detected in lymphoid and nonlymphoid tissues. Their developmental origin is still under investigation, but several results suggest that both intra- and extrathymical maturation pathways may exist.129,130 Early findings already described a non-MHC restricted-natural suppressor activity of murine DN T-cell lines,131 although cytokine analysis revealed a marked IFN-γ and TNF-α., but no IL-2, IL-4, IL-10, or IL-13 production.132,133 Meanwhile there is ample evidence of the regulatory function of DN T cells in vitro and in vivo.132–134 In contrast to naturally occurring CD4+CD25+ regulatory T cells (see Section “Functionality”), human DN T reg cells seem to exert their suppressive function in an antigen-specific fashion.133 Interestingly, the capacity of DN T reg cells to suppress syngeneic CD8+ and CD4+ effector cells arises from their Fas/Fas L-mediated cytotoxicity.134 DN T cells use their TCR complex to acquire allo-MHC peptides from APC via trogocytosis (acquisition of membrane-bound proteins) and then kill CD8+ T cells that recognize the same allo-MHC peptides.135 In vivo experiments in murine transplantation models confirmed a cell-to-cell contact-dependent, antigen-specific killing of CD8+ effectors by DN T cells that effectively prolonged skin allograft survival132 and, in addition, plays a role in preventing graft-versus-host disease.136 Similarly, a protective role of DN T cells has been proposed for autoimmune diseases137 and cancer development.136
![]() A low percentage (1%–3%) of mature CD4+CD8+ double-positive (DP) T cells can be detected in peripheral mammalian blood. They can further be distinguished based on the extent of CD4 and CD8 expression, respectively, into CD4high CD8low and CD4low CD8high T-cell subsets.138 Experiments performed in adult rats showed that DP cells represent 30%–40% of yet not fully functional T lymphocytes in peripheral lymphoid organs during fetal life with gradually decreasing numbers until reaching the low percentage seen in adulthood.139 This finding has been explained by a premature release from the thymus in the peripheral blood, where their maturation into immunocompetent single positive T cells continues. In human, it is still unclear whether the small fraction of DP T cells found in adulthood represents fully immunocompetent T cells. The fact that these cells are increased to 20% of peripheral lymphocytes in chronic viral diseases such as HIV and EBV infections points in this direction.140,141 In addition, several studies demonstrated that these cells function as antigen-specific effector memory cells that contribute to the adaptive immune response during viral infections.138,142 Recently, the occurrence of effector/memory DP T cells was also described within tumors of breast cancer143 and solid metastases of human melanoma patients.144 Analysis of their cytokine profile showed the production of Th1 and Th2 cytokines including IL-13, IL-4, TNF-α, GM-CSF, Il-2, IFN-γ, and IL-5, indicating a potential role in tumor immunity.
A low percentage (1%–3%) of mature CD4+CD8+ double-positive (DP) T cells can be detected in peripheral mammalian blood. They can further be distinguished based on the extent of CD4 and CD8 expression, respectively, into CD4high CD8low and CD4low CD8high T-cell subsets.138 Experiments performed in adult rats showed that DP cells represent 30%–40% of yet not fully functional T lymphocytes in peripheral lymphoid organs during fetal life with gradually decreasing numbers until reaching the low percentage seen in adulthood.139 This finding has been explained by a premature release from the thymus in the peripheral blood, where their maturation into immunocompetent single positive T cells continues. In human, it is still unclear whether the small fraction of DP T cells found in adulthood represents fully immunocompetent T cells. The fact that these cells are increased to 20% of peripheral lymphocytes in chronic viral diseases such as HIV and EBV infections points in this direction.140,141 In addition, several studies demonstrated that these cells function as antigen-specific effector memory cells that contribute to the adaptive immune response during viral infections.138,142 Recently, the occurrence of effector/memory DP T cells was also described within tumors of breast cancer143 and solid metastases of human melanoma patients.144 Analysis of their cytokine profile showed the production of Th1 and Th2 cytokines including IL-13, IL-4, TNF-α, GM-CSF, Il-2, IFN-γ, and IL-5, indicating a potential role in tumor immunity.
After positive selection in the thymus, mature T cells with low affinity for self-peptide/MHC molecules are released into the blood stream and form the long-lived pool of naive T cells. In order to survive, naive T cells require IL-7 signaling and a low level of self-reactivity entertained by constant TCR engagement with self-p/MHC molecules.145
![]() Recent studies identified fibroblastic reticular cells in secondary lymphoid organs as essential source of IL-7 and the CCR7 ligand CCL19.146 High expression of CCR7 and CD62L on naive T cells ensures their homing to LN and, at the same time, enables their IL-7-mediated survival. Under homeostatic conditions, a stable population size of naive T cells can thereby be maintained. The transcription factor FoxO1 has been identified as important regulator for the expression of CCR7, CD62L, and the a chain of the IL-7 receptor (CD127) on naive T cells. FoxO1-deficient mice fail to home to secondary lymphoid organs and show only very low levels of CD127, which, in turn, leads to a decrease of naive T cells in these mice.147 On robust activation, naive T cells undergo a process of expansion and differentiate into effector cells with potent pathogen-eliminating functions.148 A great proportion of effector cells dies off within a few weeks, but few cells are selected to enter the memory pool according to their capacity to access and use of survival signals.
Recent studies identified fibroblastic reticular cells in secondary lymphoid organs as essential source of IL-7 and the CCR7 ligand CCL19.146 High expression of CCR7 and CD62L on naive T cells ensures their homing to LN and, at the same time, enables their IL-7-mediated survival. Under homeostatic conditions, a stable population size of naive T cells can thereby be maintained. The transcription factor FoxO1 has been identified as important regulator for the expression of CCR7, CD62L, and the a chain of the IL-7 receptor (CD127) on naive T cells. FoxO1-deficient mice fail to home to secondary lymphoid organs and show only very low levels of CD127, which, in turn, leads to a decrease of naive T cells in these mice.147 On robust activation, naive T cells undergo a process of expansion and differentiate into effector cells with potent pathogen-eliminating functions.148 A great proportion of effector cells dies off within a few weeks, but few cells are selected to enter the memory pool according to their capacity to access and use of survival signals.
Two types of CD45RO+ memory T cells can be generated: central memory and effector memory T cells.
Similar to naive T cells, long-lived central memory T cells express the lymph node homing receptors CD62L and CCR7, which allow their circulation through peripheral blood and secondary lymphoid organs. They are responsible for secondary or long-term responses to antigen and might be involved in long-term maintenance of effector memory cells.149 The pool of memory T cells increases gradually with age at the expense of their naive counterparts. In contrast to naive T cells, memory T cells undergo cell division within an interval of 2–3 weeks, which is balanced by an almost equivalent number of cell death.150 The homeostatic expansion and survival of central memory T cells crucially depend on the responsiveness to IL-7 and IL-15, mediated via surface expression of CD127 (IL7Rα) and CD122 (IL-15R), respectively.151 Central memory T cells exhibit only modest effector functions, but, upon rechallenge with a given antigen, they can develop into effector T cells.152
![]() It appears that the strength of the antigenic signal determines the ultimate fate of a naive T cell, as robust TCR signaling may result in the generation of effector memory T cells.149 Contrary to central memory T cells, effector memory cells are excluded from secondary lymphoid organs, but home to peripheral tissues and are responsible for immediate protection against challenge. Following the peak of the immune response, most of these cells disappear from the blood and central memory T cells appear instead. It seems that effector memory cells represent a transitory population rather than a distinct cell type, ending with the development of central memory T cells.153 Conversely, central memory T cells convert into effector cells and subsequently into effector memory T cells in the presence of antigen.153 Recent studies in mice demonstrated the importance of IL-2 signaling for the survival and differentiation of long-term effector memory cells, as IL-2Rα-deficient T cells maintain the phenotype of central memory T cells and do not differentiate into effector memory T cells upon secondary antigen challenge.154 In contrast, IL-15 seems to play a negligible role in promoting effector memory differentiation during primary immune responses, but it is apparently essential for the survival of effector memory T cells after pathogen clearance.154
It appears that the strength of the antigenic signal determines the ultimate fate of a naive T cell, as robust TCR signaling may result in the generation of effector memory T cells.149 Contrary to central memory T cells, effector memory cells are excluded from secondary lymphoid organs, but home to peripheral tissues and are responsible for immediate protection against challenge. Following the peak of the immune response, most of these cells disappear from the blood and central memory T cells appear instead. It seems that effector memory cells represent a transitory population rather than a distinct cell type, ending with the development of central memory T cells.153 Conversely, central memory T cells convert into effector cells and subsequently into effector memory T cells in the presence of antigen.153 Recent studies in mice demonstrated the importance of IL-2 signaling for the survival and differentiation of long-term effector memory cells, as IL-2Rα-deficient T cells maintain the phenotype of central memory T cells and do not differentiate into effector memory T cells upon secondary antigen challenge.154 In contrast, IL-15 seems to play a negligible role in promoting effector memory differentiation during primary immune responses, but it is apparently essential for the survival of effector memory T cells after pathogen clearance.154
![]() Very recently, two new T-cell subsets have been described: Th22 (T22) cells and Th9 (T9) cells.156,157 Th22 cells were identified in human peripheral blood as skin-homing CCR6+ CCR4+ CCR10+ CLA+ memory T cells that produce IL-22, but no IL-17 or IFN-γ.156 That somehow came as a surprise, as IL-22 has so far been associated with Th17 cells only. In vivo, Th22 cells could be isolated from the epidermis of inflammatory skin diseases such as psoriasis, atopic eczema, and allergic contact dermatitis.158 In vitro, their generation from naive T cells was reported to be dependent on IL-6 and TNF-α.., but independent from the Th17-specific transcription factor RORγt.156 Th9 cells produce IL-9 upon stimulation with TGF-β and IL-4 and do not express any of the established transcription factors for T-cell differentiation, suggesting a new lineage of T cells. IL-9, originally associated with Th2-dominated responses, is known to be involved in immune responses to helminths and to contribute to the pathogenesis of asthma. Lately, is has also been described that T-cell derived IL-9 may mediate immune suppression, as it is functionally important for allograft survival.159 Future experiments will tell whether Th22 and Th9 T cells truly represent distinct T-cell subsets with lineage-specific transcription factors.
Very recently, two new T-cell subsets have been described: Th22 (T22) cells and Th9 (T9) cells.156,157 Th22 cells were identified in human peripheral blood as skin-homing CCR6+ CCR4+ CCR10+ CLA+ memory T cells that produce IL-22, but no IL-17 or IFN-γ.156 That somehow came as a surprise, as IL-22 has so far been associated with Th17 cells only. In vivo, Th22 cells could be isolated from the epidermis of inflammatory skin diseases such as psoriasis, atopic eczema, and allergic contact dermatitis.158 In vitro, their generation from naive T cells was reported to be dependent on IL-6 and TNF-α.., but independent from the Th17-specific transcription factor RORγt.156 Th9 cells produce IL-9 upon stimulation with TGF-β and IL-4 and do not express any of the established transcription factors for T-cell differentiation, suggesting a new lineage of T cells. IL-9, originally associated with Th2-dominated responses, is known to be involved in immune responses to helminths and to contribute to the pathogenesis of asthma. Lately, is has also been described that T-cell derived IL-9 may mediate immune suppression, as it is functionally important for allograft survival.159 Future experiments will tell whether Th22 and Th9 T cells truly represent distinct T-cell subsets with lineage-specific transcription factors.
With regard to the functional capacities of various T-cell subsets, it was originally assumed that CD4+ cells predominantly subserve helper functions and that CD8+ cells act as killer cells. Many exceptions to this rule are now known to exist; for example, both CD4+ and CD8+ regulatory cells are found, but CD4+ cells are still commonly referred to as helper T cells (Th cells) and CD8+ cells as cytotoxic T cells (Tc cells).
During an immune response, naive Th/Tc cells can differentiate into several functional classes of cells: (1) Th1 cells (type 1 T cells); (2) Th2 cells (type 2 T cells); (3) Th17 cells; (4) natural killer T cells (NKT); (5) regulatory T cells (T reg); and (6) T follicular helper (Tfh) cells (Fig. 10-4). Originally, all these T-cell subsets have mainly been defined as CD4+ Th cells. In the meantime we have learned that both CD4+ Th and CD8+ Tc cells can produce cytokines allowing their classification into these distinct T-cell subsets. The functional commitment of effector T-cell populations is controlled by the expression of lineage-specific transcription factors, but individual T cells can also express cytokines that are not lineage-specific. It therefore remains to be determined whether T cells display heterogeneity within a lineage or whether each distinct cytokine-expression pattern already reflects a separate lineage. It seems that T cells, although already polarized, still possess a high degree of functional plasticity that allows further differentiation depending on various factors such as the strength of antigenic signaling, cytokines, or interactions with other cells encountered in their microenvironment.155
T cells that produce IL-2, IFN-γ, and TNF are termed Th1 cells. They are the main carriers of cell-mediated immunity (CMI). Other T cells produce IL-4, IL-5, IL-6, IL-13, and IL-15. These are termed Th2 cells and are primarily responsible for extracellular immunity (see below).160,161 Many factors influence whether an uncommitted T cell develops into a mature Th1 or Th2 cell. The cytokines IL-12 and IL-4, acting through signal transducer and activator of transcription (STAT) 4 and 6, respectively, are key determinants of the outcome, as are antigen dose, level of costimulation, and genetic modifiers. Certain transcription factors have causal roles in the gene-expression programs of Th1 and Th2 cells. For example, the T-box transcription factor T-bet is centrally involved in Th1 development, inducing both transcriptional competence of the IFN-γ locus and selective responsiveness to the growth factor IL-12.162 By contrast, the zinc-finger transcription factor GATA-3 seems to be crucial for inducing certain key attributes of Th2 cells, such as the transcriptional competence of the Th2 cytokine cluster, which includes the genes encoding IL-4, IL-5, and IL-13.163,164
In murine models of intracellular infection, resistant versus susceptible immune responses appear to be regulated by these two T-cell subpopulations.165–167 Th1 cells, primarily by the release of IFN-γ, activate macrophages to kill or inhibit the growth of the pathogen and trigger cytotoxic T-cell responses, which results in mild or self-curing disease. In contrast, Th2 cells facilitate humoral responses and inhibit some cell-mediated immune responses, which results in progressive infection. These cytokine patterns are cross-regulatory. The Th1 cytokine IFN-γ downregulates Th2 responses. The Th2 cytokines IL-4 and IL-10 downregulate both Th1 responses and macrophage function. The result is that the host responds in an efficient manner to a given pathogen by making either a Th1 or Th2 response. Sometimes, the host chooses an inappropriate cytokine pattern, which results in clinical disease.
Of particular interest to immunologists is the delineation of factors that influence the T-cell cytokine pattern. The innate immune response is one important factor involved in determining the type of T-cell cytokine response.
The ability of the innate immune response to induce the development of a Th1 response is mediated by release of IL-12, a 70-kDa heterodimeric protein.168 For example, in response to various pathogens, APCs including DCs and macrophages release IL-12, which acts on NK cells to release IFN-γ. The presence of IL-12, IL-2, and IFN-γ, with the relative lack of IL-4, facilitates Th1 responses. In contrast, in response to allergens or extracellular pathogens, mast cells or basophils release IL-4, which in the absence of IFN-γ leads to differentiation of T cells along the Th2 pathway. It is intriguing to speculate that keratinocytes may also influence the nature of the T-cell cytokine response. Keratinocytes can produce IL-10, particularly after exposure to UVB radiation.96 The released IL-10 can specifically downregulate T1 responses, thus facilitating the development of Th2 responses.
Not every T-cell-mediated immune response and/or disease can be easily explained by the T1/T2 paradigm. Certain T-cell subpopulations are characterized by the secretion of IL-17. These cells are therefore termed Th17 cells. It was originally assumed that Th1 and Th17 cells arise from a common T1 precursor, but it now appears that Th17 cells are a completely separate and early lineage of effector CD4+ T cells produced directly from naive CD4+ T cells. This was proven by the identification of the Th17-specific transcription factor ROR (RAR-related orphan nuclear receptor) that regulates the expression of IL-17, IL-23R, and CCR6 in Th17 cells.170 The expression of CCR6 is unique for Th17 cells amongst T cells and regulates their migration into epithelial sites depending on its ligand CCL20.171 Recently, it has been demonstrated that Th17 cells may originate from a small subset of CD4+ T cells bearing the NK-cell-associated C-type lectin NKP-1A (CD161), which are present in cord blood and newborn thymus.172 Differentiation of human Th17 cells strongly depends on IL-23, a member of the IL-12 family, as well as on IL-1β, IL-6, and low doses of TGF-β173,174; murine Th17-lineage commitment is mainly induced by IL-6 and TGF-β. Importantly, the induction of Th17 cells from naive precursors may be inhibited by IFN-γ and IL-4, using a cross-regulatory mechanism between Th1, Th2, and Th17 cells. One of the main physiological roles of Th17 cells is to promote protection against fungi, protozoa, viruses, and various extracellular bacteria, but Th17 cells have also been linked to a growing list of autoimmune and inflammatory diseases such as neuroinflammatory disorders, asthma, lupus erythematosus, rheumatoid arthritis, Crohn’s disease and, most notably, psoriasis.99,175 Very recent evidence exists that Th17 cells might also play a role in antitumor immunity.176 Importantly, IL-17 expression is not restricted to CD4+ cells only, but has also been detected in CD8+ T cells.177 Th17 cells exert their function by producing effector cytokines including IL-17A, IL-17F, IL-22, and IL-26. Whereas IL-17 is believed to contribute to the pathogenesis of these diseases by acting as potent proinflammatory mediator, IL-22 has been described as a multifunctional cytokine with inflammatory as well as protective properties. In vitro stimulation of normal keratinocytes with IL-22, for example, results in inhibition of keratinocyte differentiation followed by epidermal hyperplasia and upregulated expression of proinflammatory genes in these cells.178
![]() NKT cells are a distinctive T-cell population with low frequency ranging from 0.01% to 1% of T cells in peripheral blood. They have properties of NK cells but, at the same time, express TCR α/β that, in human beings, consists of an invariant a chain (Vα24-Jα18) preferentially paired with a Vb11 chain. Phenotypically, NKT cells are also defined by the expression of CD45RO and CD161, indicating their effector/memory function. These cells specifically recognize certain tumor-cell-associated or bacterial glycolipids in the context of CD1 molecules and are therefore implicated in tumoricidal and bactericidal host responses (see Section “CD1-Dependent Antigen Presentation”). On antigenic stimulation, NKT cells produce large quantities of cytokines, particularly IL-4 and IL-10, and can use them to suppress Th1 responses. The biologic relevance of these in vitro data can be deduced from the observation that depletion of NKT cells can aggravate and accelerate Th1-mediated autoimmune diseases in mice, such as insulin-dependent diabetes, multiple sclerosis, and inflammatory bowel disease.180
NKT cells are a distinctive T-cell population with low frequency ranging from 0.01% to 1% of T cells in peripheral blood. They have properties of NK cells but, at the same time, express TCR α/β that, in human beings, consists of an invariant a chain (Vα24-Jα18) preferentially paired with a Vb11 chain. Phenotypically, NKT cells are also defined by the expression of CD45RO and CD161, indicating their effector/memory function. These cells specifically recognize certain tumor-cell-associated or bacterial glycolipids in the context of CD1 molecules and are therefore implicated in tumoricidal and bactericidal host responses (see Section “CD1-Dependent Antigen Presentation”). On antigenic stimulation, NKT cells produce large quantities of cytokines, particularly IL-4 and IL-10, and can use them to suppress Th1 responses. The biologic relevance of these in vitro data can be deduced from the observation that depletion of NKT cells can aggravate and accelerate Th1-mediated autoimmune diseases in mice, such as insulin-dependent diabetes, multiple sclerosis, and inflammatory bowel disease.180
An important type of immunomodulatory T cells that controls immune responses are the so-called regulatory T cells (T reg cells), formerly known as T suppressor cells.181 T reg cells are induced by immature APCs/DCs and play key roles in maintaining tolerance to self-antigens in the periphery. Loss of T reg cells is the cause of organ-specific autoimmunity in mice that results in thyroiditis, adrenalitis, oophoritis/orchitis, etc. T reg cells are also critical for controlling the magnitude and duration of immune responses to microbes. Under normal circumstances, the initial antimicrobial immune response results in the elimination of the pathogenic microorganism and is then followed by an activation of T reg cells to suppress the antimicrobial response and prevent host injury. Some microorganisms (e.g., Leishmania parasites, mycobacteria) have developed the capacity to induce an immune reaction in which the T reg component dominates the effector response. This situation prevents elimination of the microbe and results in chronic infection.
The best-characterized T reg subset is the CD4+/CD25+/CTLA-4+/GITR+ (glucocorticoid-induced TNF receptor family-related gene)/FoxP3+ lymphocytes.182 The transcription factor FoxP3 is specifically linked to the suppressor function, as evidenced by the findings that mutations in the FoxP3 gene cause the fatal autoimmune and inflammatory disorder of scurfy in mice and IPEX (immune dysregulation, polyendocrinopathy, enteropathy, X-linked) in humans. The cytokines TGF-β and IL-10 are thought to be the main mediators of suppression.
During the past years the situation has become even more complicated, because, at least under certain conditions, subsets with different phenotypes have been associated with regulatory functions such as CD4+, CD8+, and NKT cells. Accordingly, the existence of T reg cells coexpressing IL-17 and FoxP3 has been described.183 CD8+ cells can also be activated to become suppressor cells by antigenic peptides that are presented in the context of an MHC class Ib molecule [Qa1 in mice; human leukocyte antigen E (HLA-E) in humans]. CD8+ T reg cells suppress T cells that have intermediate affinity for self or foreign antigens and are primarily involved in self–nonself discrimination. In addition, recent data provides evidence for a suppressive function of human FoxP3-, TGf-β-producing γ/δ T cells.184
Tfh cells represent a distinct subset of CD4+ T cells found in limited numbers, especially in B-cell areas of lymph nodes and spleen.
Homing and long-term residency in B-cell follicles of these newly described T cells is secured by their surface expression of CXCR5. They have a crucial role in orchestrating T-cell-dependent effector and memory B-cell responses, produce IL-21 and express inducible T-cell costimulator (ICOS) and programed cell death 1 (PD-1) as costimulatory and coinhibitory molecules, respectively. Specific differentiation of Tfh cells was associated to the transcription factor Bcl6 as well as to the cytokines IL-6 and IL-21.185–187
As opposed to normal mouse skin, in which a resident population of dendritic epidermal T cells uniformly equipped with a nonpolymorphic, canonical  δ TCR exists, the lymphocytes of normal human skin are mainly located in the dermis and predominantly express the
δ TCR exists, the lymphocytes of normal human skin are mainly located in the dermis and predominantly express the  β TCR rather than the γ/δ TCR. While the majority of epidermal T cells exhibit the CD8+/CD4− phenotype, dermal T cells are mainly CD4+/CD8−, belong to the CD45RO memory population, express the addressins CLA (cutaneous lymphocyte antigen) and CCR4 which they use for skin-homing purposes,188 and are largely devoid of CCR7 and L-selectin, i.e., addressins promoting the homing of lymphocytes to the lymphoid organs.152,189 This situation is true also for homeostatic conditions which means that a cutaneous pool of effector memory cells is already in place when danger is imminent. Some of these effector memory T cells have a rather long life span and have been found in different skin conditions, for example, at sites of HSV infection of mice 190,191 and men192 as well as in clinically resolved, hyperpigmented fixed drug eruptions.193
β TCR rather than the γ/δ TCR. While the majority of epidermal T cells exhibit the CD8+/CD4− phenotype, dermal T cells are mainly CD4+/CD8−, belong to the CD45RO memory population, express the addressins CLA (cutaneous lymphocyte antigen) and CCR4 which they use for skin-homing purposes,188 and are largely devoid of CCR7 and L-selectin, i.e., addressins promoting the homing of lymphocytes to the lymphoid organs.152,189 This situation is true also for homeostatic conditions which means that a cutaneous pool of effector memory cells is already in place when danger is imminent. Some of these effector memory T cells have a rather long life span and have been found in different skin conditions, for example, at sites of HSV infection of mice 190,191 and men192 as well as in clinically resolved, hyperpigmented fixed drug eruptions.193
Normal human skin contains approximately 1 million T cells per cm2, 2%–3% of which reside within the epidermis,194 primarily in the basal and suprabasal layers. The T cells of the dermis are preferentially clustered around postcapillary venules of the superficial plexus high in the papillary dermis and are often situated just beneath the dermal–epidermal junction and within, or in close proximity to, adnexal appendages such as hair follicles and eccrine sweat ducts.
The process of T-cell trafficking to the skin is guided by a series of receptor–ligand interactions between cells. It is of note that DCs are capable of imprinting homing receptor expression on T cells,195 which means that T cells programed by skin and/or skin-derived DCs will preferentially return to the skin. One such moiety is the glycoprotein cutaneous lymphocyte antigen (CLA) that defines a subset of memory T cells that home to skin. It is a glycosylated form of P-selectin–glycoprotein ligand 1 that is expressed constitutively on all human peripheral blood T cells. The level of CLA on cells is regulated by an enzyme, α (1,3)-fucosyltransferase VII, which modifies P-selectin glycoprotein ligand 1. In this manner, CLA+ cells bind to both E-selectin and P-selectin, whereas CLA− cells bind P-selectin, but not E-selectin.196,197 The chemokine–chemokine receptor system is the other major regulator and coordinator of leukocyte migration to the skin (see Chapter 12).
Of particular importance for skin homing of memory T cells, independent of their polarization, is the interaction of CCL17 and CCL22 with CCR4 and of CCL27 with its counterreceptor CCR10 on CLA+ T cells. CCL17 is synthesized by activated keratinocytes, DCs and endothelial cells of the skin, while CCL22 is mainly of macrophage and DC origin. The CCR10 ligand, CCR27, appears to be exclusively produced by epidermal keratinocytes.198 Although it was originally assumed that functionally different T-cell subsets can be distinguished from each other by their chemokine receptor expression pattern and their responsiveness to the respective chemokines, the situation is less clear now. Reportedly, T1 cells selectively bear CXCR3 and CCR5, T2 cells preferentially exhibit CCR8 and CCR3, and T17 as well as T reg express CCR6, allowing them to respond to the keratinocyte- and endothelial cell-derived chemokine CCL20.199,200
From all that has been said so far, one can surmise that the accumulation of T cells in skin is not stochastic. This is indeed the case as exemplified by the dominance of CD8+ T cells in skin lesions, but not in the peripheral blood of patients with lepromatous leprosy201 as well as by the clonality of the T-cell population in cutaneous T-cell lymphoma, in which a single V gene usage is found to predominate in different skin lesions from the same individual.202,203 A limited TCR V gene usage has also been reported to be present in skin lesions of leprosy,204 psoriasis,205 basal cell carcinoma, and countless other reactions in which T cells are present.
The most direct indication of relevant T-cell populations in skin is determination of the number of antigen-specific T cells. It has been documented that 1 in 1,000 to 1 in 10,000 T cells in the peripheral blood, but only 1 in 50 to 1 in 100 T cells recognize the antigen causing the disease at sites of inflammation.206,207 Thus, there is as much as a 100-fold enrichment of antigen-reactive T cells at the site of cutaneous inflammation.
With regard to survival and/or expansion of T cells of human skin/epidermis, it appears that IL-2, IL-7, and IL-15 play important roles.208 Notably, the latter two T-cell growth factors can be produced by human epidermal cells, and all of them are frequently overexpressed in T cell-rich skin lesions, for example, in patients with tuberculoid leprosy. For a long period of time, the Th1/Th2 paradigm was used to explain the pathogenesis and, more often, the course of infectious, inflammatory and, even, neoplastic skin diseases. Leprosy and leishmaniasis are outstanding examples of diseases in which the clinical manifestations are decisively determined by the dominance of either Th1 or Th2 cells. With the identification of new function-based T-cell subpopulations (e.g., T0 cells, Th17 cells, Th22 cells), this classification is too rigid and no longer tenable. In fact, we come to realize that the T-cell pathogenesis of certain diseases that we had originally considered to belong into either the Th1 (e.g., psoriasis, allergic contact dermatitis) or the Th2 world (atopic dermatitis) is very complex and sometimes even stage-specific. Th17 and/or Th22 cells are apparently major players in psoriasis158 and allergic contact dermatitis.177 In atopic dermatitis, the acute lesions harbor not only Th2, but also Th17 and Th22 cells; in the chronic stage, however, Th1 cells seem to predominate. In syphilis, perhaps not only Th1 cells, but also CD8+IFN-γ-producing Th17 cells do confer immunologic resistance to T. pallidum.209,210 Th17 cells may also be important in the pathogenesis of Borrelia burgdorferi-induced Lyme arthritis, which was long attributed to be a solely Th1 cell-mediated response.211,212
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree