Fig. 17.1
Tinea corporis with the classic appearance of “ringworm”
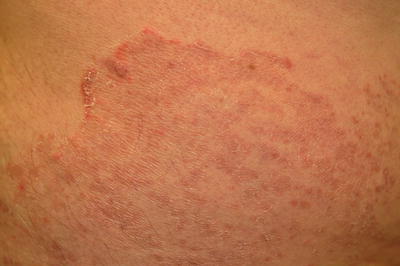
Fig. 17.2
Tinea corporis showing a serpiginous border
The differential diagnosis of tinea corporis is extremely broad, but history and a complete physical examination can usually narrow it down considerably. The list includes forms of dermatitis, such as nummular, atopic, seborrheic, and contact; papulosquamous disorders such as psoriasis, parapsoriasis, and pityriasis rosea; infections such as pityriasis (tinea) versicolor and impetigo; and autoimmune/inflammatory conditions like granuloma annulare, erythema annulare centrifugum, and subacute cutaneous lupus erythematosus. The adage, “If it scales, scrape it,” is a way to help avoid missing an easily treatable disease that can be confused for so many others.
One notable variant of tinea corporis is Majocchi granuloma, in which the organism invades the hair follicle and surrounding dermis (Fig. 17.3). This leads to the development of nodules and induration, in addition to perifollicular papules and pustules. Majocchi granuloma is most often due to T. rubrum and can be seen as a result of shaving, occlusion, or immunosuppression. Mimickers include bacterial folliculitis, eosinophilic folliculitis, acne keloidalis nuchae, Kaposi sarcoma, lymphocytoma cutis, nodular vasculitis, and crusted scabies.
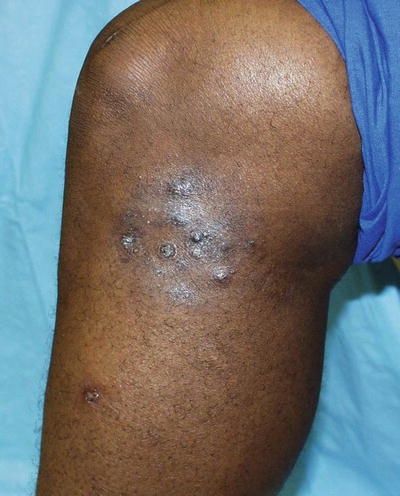

Fig. 17.3
Majocchi granuloma demonstrating follicular-based papules
Tinea Cruris
Tinea cruris, commonly known as jock itch, is a dermatophyte infection of the inner thighs and inguinal folds. It primarily affects males. The most common route of infection is local spread from concomitant tinea pedis, via clothing or hand contact. The two most common organisms identified in tinea cruris are Epidermophyton floccosum and T. rubrum [4]. Clinically, it presents as pruritic red patches or thin plaques in the upper thighs and inguinal folds. Scale may be less obvious due to the local moisture but is most likely to involve the advancing edge of the lesion. Tinea cruris characteristically does not involve the scrotum. It should be noted that cutaneous candidiasis often will involve the scrotum and should be considered in the differential diagnosis when the scrotum is involved.
The differential diagnosis of tinea cruris is rather broad and is similar to that of tinea corporis. Other cutaneous infections like candidiasis and erythrasma can be diagnosed with a potassium hydroxide (KOH) preparation, culture, or Wood lamp examination. Intertrigo is an inflammatory condition of the groin or other skin folds due to friction, occlusion, and other mechanical factors that is commonly colonized or superinfected by fungi, yeast, and bacteria. Psoriasis, seborrheic dermatitis, contact dermatitis, and lichen simplex chronicus can all involve the groin. Finally, less common entities such as mycosis fungoides, Hailey-Hailey disease, pemphigus vegetans, and Langerhans cell histiocytosis can be considered.
Tinea Manuum
Tinea manuum refers to infection of the palmar surface and interdigital areas of the hands. Infections of the dorsal hands behave like tinea corporis and can be treated as such. Tinea manuum is usually associated with tinea pedis and is therefore caused by the same organisms: E. floccosum, T. rubrum, and T. mentagrophytes. However, two nondermatophyte fungi from the genus Scytalidium can cause an infection that is identical to tinea manuum [5].
Clinically, tinea manuum presents as diffuse fine scaling of the palm with accentuation of the creases (Fig. 17.4). One or more fingernails may be involved. A classic presentation is known as “one hand, two foot syndrome,” in which tinea manuum, usually of the patient’s dominant hand, coexists with bilateral tinea pedis (Fig. 17.5). The differential diagnosis includes contact dermatitis, both irritant and allergic; atopic dermatitis; psoriasis; hyperkeratosis; and keratoderma.
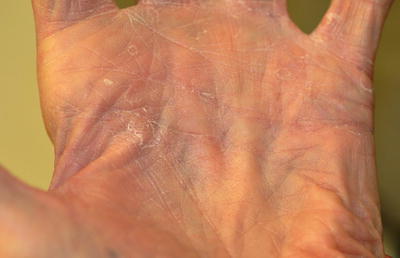
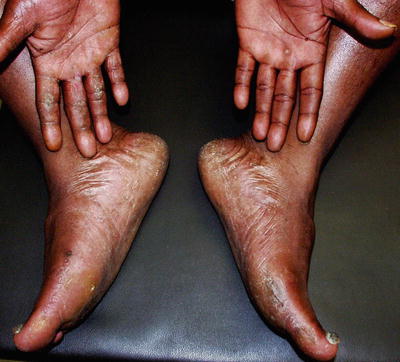

Fig. 17.4
Tinea manuum—note the accentuation of palmar creases

Fig. 17.5
“One hand, two foot” syndrome showing fine scale of the right hand and both feet; the left hand is free of disease
Tinea Faciei
Tinea faciei is a dermatophyte infection of the glabrous, or non-hair-bearing, areas of the face. When a man’s beard area is involved, it is referred to as tinea barbae. Tinea faciei and tinea barbae are most commonly acquired from pets but can also be via local spread on the same host or by person-to-person contact. The most common organisms vary by region, but T. mentagrophytes, T. tonsurans, and T. verrucosum have all been implicated.
More so than the other dermatophytoses, tinea faciei is often misdiagnosed, and therefore mistreated, for long periods of time. While it may have the classic annular plaque with a leading scale, these features are often missing due to anatomic variations of the face and frequent use of topical steroids. Contact dermatitis, seborrheic dermatitis, lupus erythematosus, periorificial dermatitis, acne, and rosacea can all be confused with tinea faciei.
Tinea barbae, due to extension down the hair follicle, tends to present as a cluster of tender, inflamed papules, pustules, and nodules which can coalesce into large boggy plaques. There may additionally be hair loss, secondary infection, lymphadenopathy, and systemic symptoms. The differential includes severe seborrheic dermatitis, contact dermatitis, psoriasis, bacterial folliculitis, pseudofolliculitis barbae, discoid lupus erythematosus, and sarcoidosis. There also exists a more superficial form of tinea barbae, usually caused by T. rubrum, which is less inflammatory and is akin to tinea faciei as described above.
Tinea Capitis
Tinea capitis refers to dermatophyte infection of the scalp and hair. While it is common in children, it is rare in the adult population. It can be caused by any Trichophyton or Microsporum species, but T. tonsurans accounts for the vast majority of cases in the United States [6]. The most common route of infection is person-to-person spread, either by direct contact or via fomites such as clothing, combs, and brushes. Animal to human spread exists but is less prevalent.
Tinea capitis can present with a wide range of clinical findings, depending on the organism involved as well as the host immune response. The most common presentation begins with perifollicular red papules that coalesce into scaly plaques. Pustules, crusting, posterior cervical lymphadenopathy, and varying amounts of pruritus may be present. Alopecia is often present and may be the most prominent finding. T. tonsurans, an endothrix organism, can invade the hair shaft and weakens it. The shaft then breaks at the scalp surface, leading to the so-called “black dot” tinea capitis. Zoonotic organisms such as M. canis tend to cause more of an inflammatory response from the host, which can lead to large boggy plaques called kerions. These are commonly associated with lymphadenopathy and systemic findings, and they tend to heal with scarring and hair loss.
The differential diagnosis of tinea capitis usually includes psoriasis and seborrheic dermatitis, which can both cause significant erythema and scale. If scale is less prominent, alopecia areata and trichotillomania can be considered. Other entities in the differential include lichen planopilaris, discoid lupus, acne keloidalis nuchae, pyoderma, and folliculitis, including folliculitis decalvans and dissecting cellulitis. It is often taught that any child with a scaly scalp deserves a fungal culture to rule out tinea capitis.
Tinea Pedis
Tinea pedis, commonly known as athlete’s foot, is a dermatophyte infection of the plantar surface and interdigital spaces of the feet. It is the most common dermatophytosis on the planet. It most often affects adult males, though infection in females is not rare. Risk factors for tinea pedis include hot, humid environments and use of occlusive footwear. Direct contact with communal areas like pools and locker rooms is likely a source of infection. The most common organisms that cause tinea pedis are T. rubrum and E. floccosum [4].
Tinea pedis usually presents as faint erythema and fine white scale of the toe webs and plantar surface that extends slightly onto the sides of the feet. This presentation is often referred to as “moccasin distribution” (Fig. 17.6). As discussed above, one hand may also be involved in the “one hand, two foot syndrome.” Another presentation is bullous tinea pedis, which consists of multiple tense vesicles and bullae on the distal feet and between toes. This is most often caused by T. mentagrophytes.
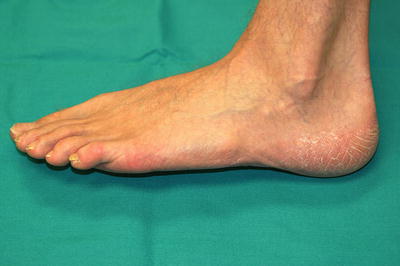

Fig. 17.6
Tinea pedis with a moccasin distribution
The differential diagnosis of tinea pedis includes infection of nondermatophyte fungi from the genus Scytalidium, eczema, contact dermatitis, psoriasis, palmoplantar pustulosis, secondary syphilis, erythrasma, and bacterial infection. Mixed toe web infection is a polymicrobial infection of the distal foot and toe webs that may be a complication of tinea pedis, as this allows a portal of entry. Gram negative organisms, including Pseudomonas, are commonly implicated. Clinically this appears as severe maceration of the web spaces with profuse malodorous discharge (Fig. 17.7).
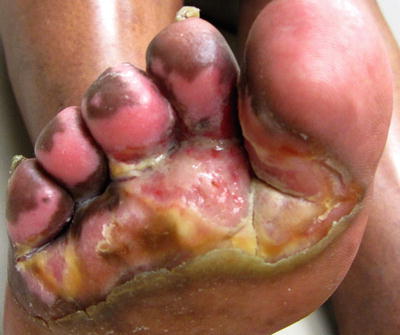

Fig. 17.7
Mixed toe web infection with severe maceration of the toe webs
Tinea Unguium
Tinea unguium, or onychomycosis, is an infection of the nail plate. Like tinea pedis, it is very prevalent, and the two are often associated. The most common causative agents in North America and much of Europe are T. rubrum, E. floccosum, and T. mentagrophytes, though any dermatophyte can be involved. Dermatophytes are responsible for up to 90 % of toenail onychomycosis, but Candida species commonly cause fingernail infection. Nondermatophyte molds must also be included in the differential diagnosis of nail dystrophy [7]. The hallmark of onychomycosis is nail dystrophy. This often consists of discoloration of the nail plate, onycholysis (separation of the nail plate from the nail bed), and the presence of chalky subungual debris (Fig. 17.8).
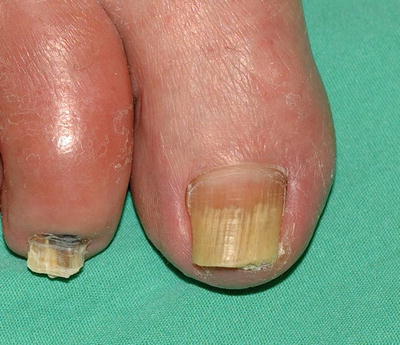

Fig. 17.8
Distal onychomycosis demonstrating onycholysis and subungual debris
There are two varieties of tinea unguium that have special implications. Proximal subungual onychomycosis, as the name suggests, involves only the proximal portion of the nail plate, and it is frequently associated with human immunodeficiency virus (HIV) infection [8] (Fig. 17.9). Superficial white onychomycosis, which typically stays in the dorsal portion of the nail plate, can be associated with T. mentagrophytes and nondermatophytes like Aspergillus and Fusarium.
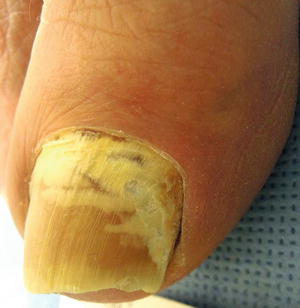

Fig. 17.9
Proximal subungual onychomycosis associated with immunosuppression
The differential diagnosis of tinea unguium includes trauma, psoriasis, atopic dermatitis, subungual tumors, lichen planus, alopecia areata, and contact dermatitis.
Nondermatophytes
Piedra
There are two forms of piedra—white and black—which are both superficial infections of the hair shaft. White piedra, which is common in temperate climates, is primarily caused by Trichosporon asahii, formerly known as Trichosporon beigelii. White piedra presents as asymptomatic, soft, white or cream colored nodules that are loosely adherent to hair shafts. While this is primarily a self-limited infection, T. asahii has been known to cause a systemic infection called trichosporonosis in immunosuppressed patients [9]. This is a multisystem disease that includes fevers, renal failure, pulmonary infiltrates, and skin lesions that range from papules to necrotic nodules.
Black piedra is caused by Piedraiahortai, and it occurs only in tropical climates. It presents as tiny, firm black nodules on the hair shafts of the scalp. It is usually asymptomatic but may result in breakage of the hair shafts. Black piedra is not known to cause systemic disease.
The differential of piedra includes pediculosis (lice), hair casts, trichomycosis axillaris, eczema, and psoriasis.
Tinea Nigra
Tinea nigra is a superficial infection caused by Hortaea werneckii. It is found in tropical climates, which can include portions of the United States. It is usually found in soil, compost, or decaying wood and vegetation. Infection typically presents as an asymptomatic brown macule or patch on the hand or wrist, though it may also appear in other places on the body. The major item in the differential is acral melanocytic nevus, though malignant melanoma, fixed drug eruption, postinflammatory hyperpigmentation, and external stains can also be considered.
Tinea Versicolor
Tinea versicolor is caused by lipophilic yeasts in the Malassezia genus, including M. furfur and M. globosa. These are present in small numbers on normal skin, and they cause skin disease only when they proliferate and form hyphae. This is usually stimulated by excessive heat and perspiration, but a number of other factors have been implicated [10]. Tinea versicolor occurs worldwide, with a higher incidence in hot, humid climates.
Clinically, tinea versicolor presents as numerous ovoid patches with fine scale that may coalesce. They can range in color from hypopigmented to red to hyperpigmented, depending on the host. Common areas of involvement are the back, chest, and shoulders (Fig. 17.10). Pruritus is usually absent or minimal. The differential diagnosis includes vitiligo, pityriasis alba, postinflammatory pigment alteration, seborrheic dermatitis, pityriasis rosea, guttate psoriasis, tinea corporis, and secondary syphilis.
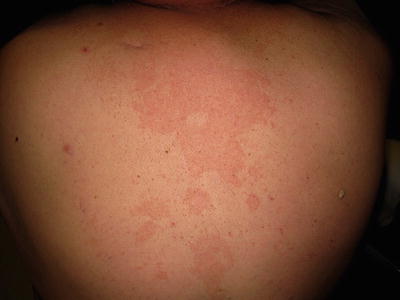

Fig. 17.10
Tinea (pityriasis) versicolor
M. furfur and M. globosa can also invade hair follicles, leading to pityrosporum folliculitis. Common in young men and women, this presents as monomorphic red papules and pustules on the upper trunk and shoulders. It can be pruritic. Pityrosporum folliculitis has also been known to arise in the immunosuppressed and those on chronic antibiotics. The differential commonly includes acne, bacterial folliculitis, and eosinophilic folliculitis.
Mucocutaneous Candidiasis
Mucocutaneous candidal infections can range from benign self-limited infections such as angular cheilitis to chronic and debilitating infections in those with certain immunodeficiencies. Candida albicans is the most frequent cause, followed by C. tropicalis. Mucosal candidiasis can present in a number of ways but classically resembles thick, cheesy white plaques that bleed if removed. Cutaneous candidal infections consist of bright red, weepy patches and plaques with satellite pustules. These occur most often in flexural areas such as the groin and inframammary areas. Risk factors for mucocutaneous candidal infections include diabetes, immunosuppression including HIV, antibiotic use, corticosteroids, and habitus. The differential for mucocutaneous candidiasis depends on the site(s) involved but includes seborrheic dermatitis, psoriasis, intertrigo, dermatophytosis, contact dermatitis, leukoplakia, and lichen planus, as well as many others.
The term chronic mucocutaneous candidiasis encompasses a number of immunodeficiencies and other disorders whose end result is severe and recalcitrant candidal infection. Invasive candidiasis will be discussed separately.
Subcutaneous Fungal Infections
Chromoblastomycosis
Chromoblastomycosis is caused by a number of dematiaceous fungi that are naturally found in soil and decaying plant matter. Fonsecaea pedrosoi is the most common agent, but others in the genera Fonsecaea, Phialophora, Cladophialophora, and Rhinocladiella have been identified [11]. These are most often found in tropical and subtropical climates, but they are occasionally seen in the United States. The most common route of infection is local inoculation via trauma. The first lesion is often a small verrucous papule which can slowly grow and spread locally. Over time, large, fungating tumors may develop. These can be complicated by superinfection, regional lymphadenopathy, and loss of function of the affected limb, but they rarely spread distally or to internal organs.
The differential diagnosis for chromoblastomycosis includes other subcutaneous fungal infections such as sporotrichosis and mycetoma, blastomycosis, cutaneous tuberculosis or other atypical mycobacterium, leishmaniasis, tertiary syphilis, warts, and mycosis fungoides.
Sporotrichosis
Sporotrichosis is caused by the dimorphic fungus Sporothrix schenckii. It is present in soil and on vegetation such as rose bushes and sphagnum moss. It has a broad geographic distribution which includes portions of the southeastern United States. The organism is introduced to humans almost exclusively through puncture injuries, which are usually occupation related. Sporotrichosis begins as a small red papule or nodule at the site of injury. Within weeks it will exhibit lymphangitic, or “sporotrichoid,” spread. This includes the development of erythematous nodules along the course of lymphatic drainage which may ulcerate (Fig. 17.11). Other forms of sporotrichosis exist, such as a fixed cutaneous form without lymphangitic spread, rosacea-like lesions, and disseminated disease [12]. However, these are the exception to the rule.
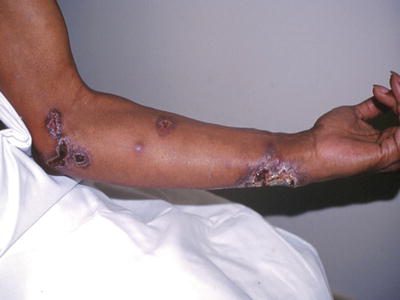

Fig. 17.11
Sporotrichosis. Note the spread of lesions along the local lymphatics
The differential diagnosis of sporotrichosis, especially with lymphangitic spread, includes the following: leishmaniasis, atypical mycobacteria, tuberculosis, nocardia, tularemia, lepromatous leprosy, and other deep and subcutaneous fungal infections.
Mycetoma
Mycetoma, or Madura foot, is a phenotype that is caused by two different classes of organisms: true fungi and filamentous bacteria. Eumycetomas, those caused by true fungi, include members of the Madurella, Pseudallescheria, Acremonium, and several other genera [13]. Actinomycetomas are caused by Nocardia, Actinomadura, Actinomyces, and Streptomyces species of bacteria. Both kinds of mycetoma are acquired through direct penetration of the organism into the foot or leg from soil. They are also found in similar environments—arid climates near the equator.
Mycetomas, both eumycotic and actinomycotic, are characterized by their draining sinus tracts and profound local edema. The lesions begin as asymptomatic nodules at the site of inoculation, before undergoing a period of steady swelling and growth. They can involve underlying structures such as fascia, muscle, and even bone, while generally remaining painless. The material that drains from the sinuses is composed of characteristic, tightly packed clumps of organisms. While the appearance of mycetoma is fairly characteristic, other subcutaneous and deep infections can be in the differential.
Opportunistic Fungal Infections with Cutaneous Lesions
Systemic Candidiasis
In immunocompromised patients, such as recipients of renal transplants, candidal infections represent the most common systemic fungal infection. The most common pathogen is C. albicans, while C. tropicalis may be more common in patients with hematologic malignancy. The portal of entry is thought to be the gastrointestinal tract, and long-term IV catheterization is a strong risk factor. The mortality associated with disseminated candidiasis is between 30 and 40 %, so early recognition and initiation of treatment is paramount [14].
Disseminated candidiasis usually presents as fever that is unresponsive to antimicrobials, proximal muscle tenderness, and skin lesions that can range from red macules and papules with a pale center, to purpura, to vesicles and necrotic ulcers. Septic shock and failure of virtually any end organ can occur. Unfortunately blood cultures will not always be positive for yeast, so a high index of suspicion is required. Items in the differential diagnosis include bacterial sepsis or septic shock, as well as other opportunistic infections such as aspergillosis, fusariosis, cryptococcosis, and phaeohyphomycosis.
Aspergillosis
Aspergillosis is an opportunistic fungal infection caused by two Aspergillus species, flavus and fumigatus. Specific risk factors for aspergillosis include neutropenia, burn patients, and high-dose corticosteroids [15]. There are two main ways in which this infection occurs. In the first, the fungus gains entry to the skin and subcutaneous tissue through a burn site, long-standing IV catheter, or compromised wound under occlusion. In an immunocompromised host, it can then invade the local vasculature and become disseminated. The second pathway is via inhalation, which leads to pulmonary infection and dissemination from there.
Cutaneous lesions in aspergillosis usually include purpura, necrotic papules, and ulcers. Disseminated aspergillosis is rapidly fatal, with spread to the lungs, kidneys, heart, and central nervous system (CNS). Septic shock and hemoptysis are frequently present.
Another form of aspergillosis is known as otomycosis, and it represents colonization or infection of the external ear canal. Concomitant infection with bacteria such as Pseudomonas aeruginosa is quite common. While this can be perfectly asymptomatic in a healthy host, in the immunocompromised, a progressive and invasive infection may occur that requires prompt parenteral antimicrobial therapy.
Zygomycosis
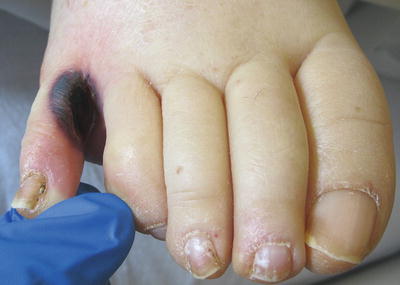
Zygomycosis is an opportunistic, angioinvasive infection caused by multiple organisms including Rhizopus and Mucor species. These fungi are ubiquitous in soil and vegetation but rarely cause disease in immunocompetent hosts. However, they are known to cause rapidly fatal infections in those with leukopenia and acidosis, particularly uncontrolled diabetes [16]. Other predisposing conditions include hematologic malignancy, pharmacologic immunosuppression, solid organ or bone marrow transplantation, burns, trauma, malnutrition, and IV drug use. Of particular interest are infections in patients with iron or aluminum overload, patients undergoing hemodialysis, and those receiving the chelating agent deferoxamine [17]. The classic and most common form of zygomycosis is rhinocerebral, which usually occurs in patients with diabetic ketoacidosis (DKA). Patients may complain of fever and unilateral facial pain, including pain with eye movements. Diffuse erythema and induration will progress to necrotic ulcers with characteristic black centers. A less common form of cutaneous zygomycosis follows trauma and direct inoculation into the skin. This can occur anywhere and usually presents as a sharply demarcated necrotic ulcer with a dark black center (Fig. 17.12). Occasionally these lesions can also occur in various locations on the skin secondary to disseminated disease. Disseminated zygomycosis is a rapidly progressive infection that usually begins in the lungs and spreads to the brain. Patients begin with fever and headaches and soon develop confusion, obtundation, and death. They may or may not have cutaneous findings. In 2008, Reyes et al. [17] reported such a case in a patient with secondary iron overload treated with deferoxamine.
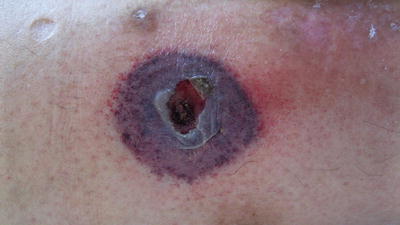

Fig. 17.12
Mucormycosis in an immunocompromised patient—note the sharply demarcated necrotic ulcer. (Courtesy of Alex G. Ortega-Loayza, M.D.)
Phaeohyphomycosis
The term phaeohyphomycosis encompasses a large group of dematiaceous fungi with this in common—the production of dark brown or black hyphae in tissue. Most of these organisms are found in soil and plant material, and they are introduced into human hosts via trauma. Phaeohyphomycosis can range from asymptomatic superficial infection, such as tinea nigra, to onychomycosis, to rapidly progressive and fatal systemic infection [18]. Infected puncture wounds can lead to cutaneous papules, subcutaneous cysts, or nodules (Fig. 17.13). In a normal host these lesions typically become walled off but do not spread further. In immunocompromised hosts, these fungi can gain access to the bloodstream and disseminate from there. Common targets of dissemination include the heart valves and central nervous system (CNS), and when present, skin lesions appear as necrotic, dry ulcers and eschars with red borders.
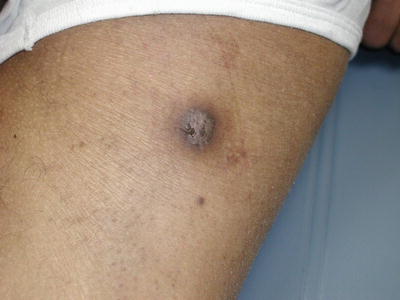

Fig. 17.13
Phaeohyphomycosis. Exophiala jeanselmei is a recognized causative agent of mycetoma and phaeohyphomycosis. The clinical presentation can be nonspecific; tissue culture is necessary to make the diagnosis. (Courtesy of Julia R. Nunley, M.D.)
Hyalohyphomycosis
Hyalohyphomycosis includes nondematiaceous fungi that produce tan or white hyphae in tissue. The most important organisms in this group include species of three genera: Fusarium, Penicillium, and Paecilomyces. These organisms are ubiquitous in nature but rarely cause disease in competent hosts. One exception is that Fusarium spp. can cause onychomycosis, which is thought to be a possible source of disseminated disease [19]. Fusarium spp. are known to affect patients with profound neutropenia, hematologic malignancy, and severe burns. While Penicillium species are mostly found in southeast Asia, they have been seen in the United States, primarily in HIV patients [20].
Clinically, patients with disseminated fusariosis will have fevers, severe myalgias, and diffuse targetoid lesions that become necrotic and ulcerated (Figs. 17.14 and 17.15). Skin findings associated with penicilliosis include papules on the forehead and cheeks that resemble acne or molluscum. The differential diagnosis of fusariosis includes other angioinvasive infections like aspergillosis and candidiasis. For penicilliosis, the list is similar but also includes dimorphic fungi like cryptococcosis, blastomycosis, histoplasmosis, and coccidioidomycosis, which can all form molluscum-type lesions in immunocompromised hosts.