Management of infantile hemangiomas includes a combination of observation, medical therapy, laser treatments, and surgery. The nomenclature to describe these lesions has been standardized and should be adhered to. The goal of treatment is to obtain the best possible result commensurate with known developmental milestones. Current knowledge of the biology of these tumors as well as experience allows obtaining this goal. “Leave it alone, it will go away” is no longer universally acceptable advice for treatment of infantile hemangiomas.
Key points
- •
Management of infantile hemangioma (IH) includes a combination of observation, medical therapy, laser treatments, and surgery.
- •
The nomenclature to describe these lesions has been standardized and should be adhered to.
- •
The goal of treatment is to obtain the best possible result commensurate with known developmental milestones.
- •
Current knowledge of the biology of these tumors as well as experience allows obtaining this goal.
- •
“Leave it alone, it will go away” is no longer universally acceptable advice for treatment of IH.
Infantile hemangioma (IH) is a vascular anomaly and the most common benign tumor of infancy. In spite of it being so prevalent, there is still a widespread lack of understanding in the medical community leading to mismanagement of affected children. The purpose of this article is to review the current knowledge on pathogenesis, diagnosis, and management of IH.
Vascular anomalies are a group of disorders that are categorized as either tumors or malformations according to the accepted classification of the International Society for the Study of Vascular Anomalies (April 2014; http://www.issva.org ). The tumors are further divided into benign, locally aggressive, or malignant entities. IH is one of the benign vascular tumors along with congenital hemangiomas, pyogenic granulomas, and a few other less common entities.
IH are true neoplasms of endothelial cell origin exhibiting up-regulated cell growth, increased mitosis, and cellular hyperplasia. Endothelial cells in IH have a clonal origin but the exact source of the progenitor cell is not clear. Striking commonality in the mRNA transcriptome have been found between IH and placental tissue, which suggests that progenitor cells from placenta may be associated with these tumors. In addition to morphologic similarities between endothelial cells of IH and placenta, IH uniquely coexpresses glucose transporter protein 1 (GLUT-1) and other markers with placenta. The only vascular anomaly that expresses GLUT-1 is IH, making this an important marker to histologically confirm a diagnosis of IH and distinguish from all other vascular lesions in the occasional case requiring a tissue biopsy. Endothelial and mesenchymal progenitor cells have been identified in both IH and placenta but the exact source of these progenitor cells is not clear. One theory suggests that fetal angioblasts differentiate into a placental vascular phenotype at locations that are prepared by circulating factors secreted by the placenta thus making the predisposed sites fertile ground for the growth of IH. This metastatic niche theory has been shown to be true in some cancers. The other theory posits that embolic progenitor cells from the placenta deposit in the developing fetus and differentiate into IH. These embolic cells may be more likely to deposit in the head and neck due to the increased vascularity in this region with the sites of predilection occurring at the end arteries of the developing facial placodes. Neither theory has yet to been proven or explains the peculiar natural history of IH, which consists of a period of rapid postnatal proliferation followed by a phase of involution.
Histologically, during proliferation, IH is characterized by a high mitotic activity in the endothelial cells and pericytes as the tumor enlarges. Involution is accompanied by an increase in apoptosis, endothelial cells flatten with enlargement of their lumina, and the lesions become dominated by fibrofatty stroma. Alterations in several cytokines important in angiogenesis have been demonstrated during the various phases. The proliferation phase is dominated by vascular endothelial growth factor (VEGF), which is a primary mitogen for benign and malignant vascular tumors and promotes cell survival while inhibiting apoptosis. Serum levels of VEGF are elevated in infants with proliferating IH compared with involuting IH and controls. VEGF activates angiogenesis via the mammalian target of rapamycin (mTOR) signaling pathway. Also elevated during this time are basic fibroblast growth factor (bFGF), insulin-like growth factor (IGF)-2, matrix metalloproteinase (MMP)-9, and type IV collagenase, whereas levels of endogenous interferon are decreased. During involution, levels of VEGF, bFGF, and IGF-2 decline, whereas levels of regulatory cytokines such as interferon and tissue inhibitor of MMP-1 (TIMP-1) increase. These factors and pathways are potential targets for clinical intervention.
Clinically, these hallmark phases form the basis for diagnosis and management. IH are typically not visible at birth; however, up to 30% are evident as precursor lesions with variable findings including a telangiectatic macule, pale vasoconstrictor area, vascular stain, or bruised appearance. Within the first weeks of life, IH becomes visible as an erythematous macule or slightly raised papular lesion. The lesions then undergo a classic progression of rapid proliferative growth followed by involution that is variable in length and extent. The period of most rapid growth occurs in the early proliferative stage and is largely complete by about 4 to 6 months of age with tumors reaching roughly 80% of their final size at this point. Despite the increase in size during proliferation, IH tend not to expand beyond the defined anatomic site of the original lesion. The late proliferative phase is complete by 9 months in most children with very little growth occurring after this point as the lesion enters the plateau phase. Involution begins as early as 6 months and may last for several years. Clinically, lesions become lighter in color and softer to palpation with diminution in the volume of the mass. If one includes all IH (scalp to soles of feet) nearly 60% of IH will involute to aesthetically and functionally acceptable endpoints; however, 40% of lesions leave a remnant that may require further treatment. They may appear as hypopigmented or telangiectatic macules, with loose, expanded soft tissue, and/or fibrofatty residual masses, depending on the nature of the original tumor. The threshold for what is considered acceptable varies with location and size. For example, a small focal lesion of the nasal tip will have a very different impact than a larger, segmental lesion of the lower back. For this reason, even though 100% of IH involute, a large number of patients will seek improvement as the threshold for acceptability is high because most occur in the face and head or neck areas ( Fig. 1 ).
Several risk factors for the development of IH have been identified. Fair-skinned individuals are at higher risk with rates up to 10% among white infants. Prematurity is associated with IH and the number of hemangiomas per infant increases with younger gestational age. Female children are more likely to develop IH than male children (3:1); however, this is less pronounced (1.8:1) among premature infants. Low birth weight, especially less than 1500 g, increases the likelihood of IH independent of gestational age. Every 500 g reduction in birth weight imparts a 40% increased risk of IH. A definite genetic link or pattern of inheritance has yet to be established with most IHs thought to be sporadic events.
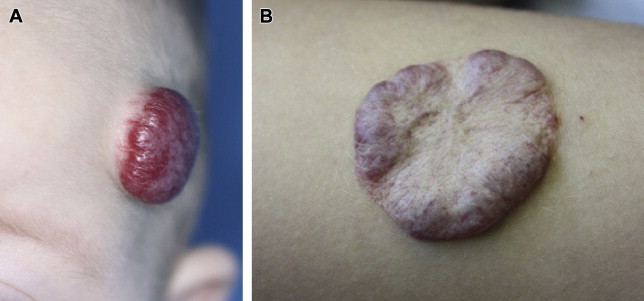
Adhering to standardized nomenclature is important to be able to communicate clinically relevant information. Terms such as cavernous hemangioma or strawberry should be abandoned as anachronisms. In addition to the phase of its natural history and anatomic site, IHs are described by the degree of cutaneous involvement. Superficial IHs are limited to the superficial dermis though they can be thick. Deep IHs involve the deep dermis and subcutaneous layers and present as masses without overlying skin involvement. Compound IHs involve both the superficial and deep layers ( Fig. 2 ).
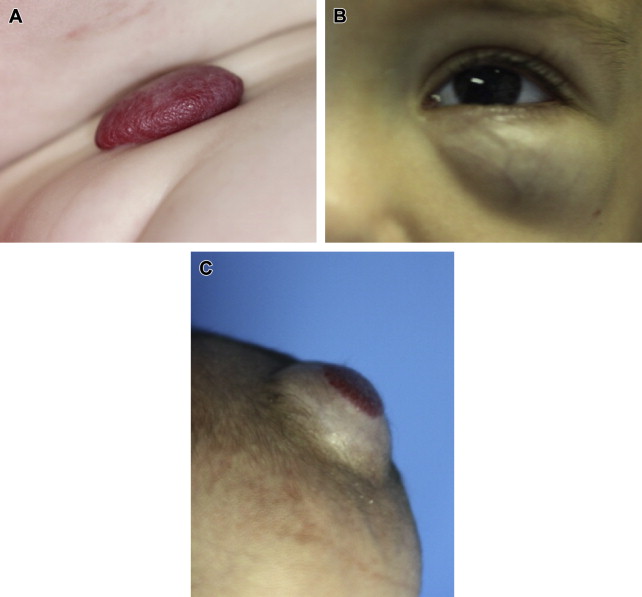
Additionally, IH are classified morphologically into (1) focal when a solitary lesion exists, (2) multifocal when multiple lesions are present, and (3) segmental when a lesion or cluster of lesions corresponds to a developmental subunit or dermatome. Focal lesions are the most common, occurring in 80% of affected patients. Although IHs can occur throughout the body, they most commonly affect the skin of the head and neck (50%–60%), followed by the trunk (25%), and limbs (15%). Facial lesions are disproportionally distributed in the central face along lines of embryonic fusion with 60% of lesions occurring in the periorbital, nasal, and perioral zones. The presence of more than five multifocal cutaneous lesions increases the chances of visceral organ involvement, particularly the liver, and further evaluation should be considered. Segmental IH are the least common subtype but are the most likely associated with other abnormalities, more difficult to treat, and have worse outcomes. These lesions loosely follow a dermatomal distribution. Segmental lesions have an even stronger predilection for involvement of the face than localized lesions and they are more common in nonwhite Hispanic infants at older gestational age and higher weight than localized lesions, suggesting there may be differences in pathogenesis. There is nearly twice the rate of female predominance among segmental IH (5.7–6.6:1) compared with the localized form (3:1). Segmental lesions of the so-called beard distribution or V3 dermatomal distribution have a unique association with IH in the airway. Most commonly this involves the subglottis. Conversely, more than 50% of patients with IH of the airway have a concomitant cutaneous IH. Segmental IH have a higher association with syndromes than focal IH. Lumbosacral segmental IH may be associated with underlying spinal dysraphia and urologic abnormalities. Beard distribution lesions also have an association with PHACE syndrome defined by the presence of a facial IH and one or more of the following: posterior fossa brain abnormalities, arterial anomalies, aortic coarctation or cardiac abnormalities, and eye anomalies. PHACE syndrome occurs in 20% to 33% of patients with segmental IH and nearly 50% of patients with airway IH but is much less common in infants with focal IHs.
The diagnostic workup for IH is simple because most cases can be diagnosed by history and physical examination. Imaging studies may be useful to evaluate for concomitant lesions in cases of greater than five visible cutaneous lesions, to assess the full extent of a lesion for surgical planning, and occasionally to lend support to an uncertain diagnosis. Ultrasonography can be helpful to examine for hepatic or abdominal IH in patients with multiple cutaneous lesions or to initially evaluate the spine in patients with overlying lumbosacral lesions. Children with concern for PHACE syndrome should undergo imaging of the brain with MRI to evaluate for posterior fossa lesions, echocardiogram, and possibly angiography or MR angiography to evaluate for aortic and cerebrovascular anomalies. When imaging is desired to determine the extent of the tumor, contrast-enhanced MRI is the modality of choice and provides excellent evaluation of soft tissue without exposing the patient to ionizing radiation. On MRI, proliferating IH appear as a distinct, lobulated, enhancing soft tissue mass that is isointense to muscle on T1 and hyper-intense on T2 images, often with a visible feeding artery and draining veins and intralesional flow voids. Involuting IH are heterogeneous masses with areas of increased T1 intensity corresponding to fibrofatty tissue and less robust enhancement than proliferating lesions. Again, imaging studies are rarely needed for the diagnosis and treatment planning of most uncomplicated IH. Uncommonly, tissue biopsy may be necessary to confirm the suspected diagnosis or exclude other entities. IH can be positively identified based on characteristic histologic appearance and positive staining for GLUT-1, which is unique to IH among all other vascular anomalies. Laboratory blood tests play no role in the evaluation of patients with IH because there is no associated coagulopathy with this condition. As with imaging, biopsy and laboratory testing is not usually needed for uncomplicated IH.
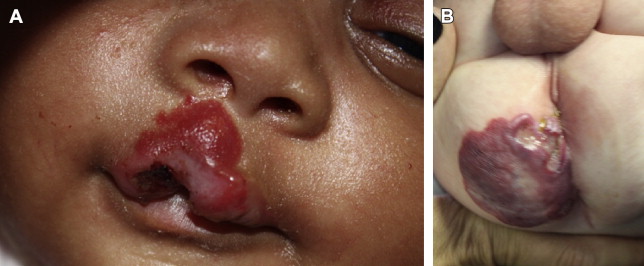
Segmental lesions are more likely to develop complications than focal ones, with up to 50% of segmental lesions undergoing ulceration compared with 10% to 15% of localized lesions. Ulceration is the most common complication occurring in about 10% of cases overall almost all occur during proliferation. The cheeks, lip, and scalp are the most likely sites to ulcerate on the face, whereas the perineal area is the most common of the rest of the body ( Fig. 3 ). Higher rates of complications (1.7 times) are seen among IH located on the face compared with other body sites and are more likely to require treatment. Functional impairment most often interferes with visual axis, feeding ability, or respiration. Complications are more likely based on size, location, and morphology of the lesions but seem unrelated with patient characteristics or demographics. Larger lesions are more likely to develop complications, particularly ulceration, with a 5% increase in complications for every 10 cm 2 increase in size. Mixed IHs are more prone to ulceration than superficial lesions. The cause of ulceration is unknown but the speculation is that rapid expansion during proliferation exceeds the elastic capacity of the skin and the lesions outgrow their blood supply leading to ulceration. However, a small minority of lesions ulcerate during involution. Ulcerated lesions may become secondarily infected, are at higher risk for bleeding, and are more likely to result in scarring or require treatment.