1. Normally high vulvar transepidermal water loss
2. Normally high vulvar friction coefficient
3. Naturally high vulvar skin hydration
4. Persistent exposure to environmental irritants, rubbing, etc
5. Tendency for spongiotic dermatitis
6. Further barrier compromise with secondary yeast infection
19.4 Recognizing the Vulvar Sensitive Skin Syndrome
The vulva is potentially sensitive to a wider range of irritants and allergens than other skin sites [16]. Sensitive skin syndrome on a woman’s face may only reflect a transient reaction to cosmetics. In contrast, vulvar sensitivity is generally an ongoing, uninterrupted reaction to urine, abrasion from clothing or contact with tight fitting clothing, hard water, harsh toilet paper, pads or panty liners, and various medicated creams and lubricants. Unfortunately, these adverse vulvar insults are generally persistent and unavoidable, in contrast to the occasional use of cosmetics on the face. Adverse cosmetics on the face can be discontinued. Thus, the dermatologic concept of sensitive skin syndrome may actually have more significant application to the vulva than to other body sites. Considering the unfortunate disability associated with the resulting vulvar pain, the reasonably well-defined concepts that describe the dermatologic sensitive skin syndrome have great importance when applied to vulvar care. It is clinically significant and scientifically instructive to view vulvar itching, burning, and pain within the domain of the sensitive skin syndrome. As acknowledgment of the unique sensitivity of the vulva, it would be appropriate at least to recognize a dermatologic subcategory of vulvar sensitive skin syndrome. A specific vulvar sensitive skin syndrome is not currently identified by dermatologists or by gynecologists as a unique diagnosis. Dermatologists graciously accept the gynecologist’s vulvodynia work as a separate entity, and gynecologists generally may not be aware of the sensitive skin syndrome in the dermatologist’s realm. With this overlap in concepts, it is appropriate to view the dermatologist’s sensitive skin syndrome as the equal counterpart to the gynecologist’s vestibulitis and vulvodynia. Gynecologists have made some progress in refining the definitions of the vulvodynia and vestibulitis concepts, but a physical explanation and an adequate treatment remain evasive in the gynecology world. Fortunately, with the application of dermatopathology and a solid understanding of skin physiology, dermatologists are achieving significant progress in the area of sensitive skin research. If the concepts of vulvodynia and vestibulitis had never been established, it is likely that a dermatologic concept of vulvar sensitive skin syndrome would adequately address these gynecologic needs.
Organized research in vulvar sensitive and painful skin started in 1976 [17] for gynecologists, and sensitive skin research independently began prior to that same era for dermatologists. Definitions can be a challenge in both categories. Causation that may be difficult to confirm occasionally must be presumed, such as allodynia (neuropathic pain). Typical of the clinical challenge of sensitive skin diagnosis, allodynia is a diagnosis of exclusion. In rare cases, a direct clinical cause for neuropathic pain, such as trauma, is evident. A biopsy of sensitive skin is useful. Vulvar skin sensitivity that would be attributed otherwise to the generic concepts of vulvodynia or vestibulitis often shows dermatologic disease if evaluated by a dermatopathologist, rather than a surgical pathologist [4]. Advances in vulvodynia and vestibulitis have been handicapped by omission of dermatopathology. Vulvar biopsy may not be the first step for diagnosis, but it becomes helpful if therapy for the presumed clinical diagnosis fails. For cancer surveillance, it is important to biopsy any persistent vulvar ulcer, erosion, or white patch.
19.5 Vulvar Spongiotic Dermatitis and Yeast Infection
Toilet paper, rubbing of clothing, various medicated creams and spermicides, etc., can be constant, essentially unavoidable irritants to the vulvar epithelium. Considering the persistent exposure to potential irritants and the fragile nature of the vulvar epithelium, a high prevalence of spongiotic dermatitis would be expected. Actually, spongiotic dermatitis is a common finding throughout the lower female genital tract. A “reactive change” reported on a pap smear reflects spongiotic change on the cervix if a biopsy is obtained [18, 19]. This finding refers to flakes of epithelium in the Pap smear sample, associated with the spongiotic change. Small flakes of epithelium are a common asymptomatic finding in the vaginal saline wet preparation (Fig. 19.1), often numerous and thick in women with sensitive skin [20] (Fig. 19.2). It would be expected that all women will experience some degree of spongiotic vulvar dermatitis at one time or another. For some women with moderately fragile vulvar epithelium, this would be a common problem. For others it can be a persistent and severe problem, depending upon relative innate factors that influence fragile skin as well as the relative harshness of the environmental conditions. Flakes of desquamated epithelium in the vaginal saline wet preparation can be viewed as a marker for spongiotic change in the female genital area. Flaking skin compromises the vulvovaginal skin barrier, facilitates microbe adhesion, and further increases sensitivity to environmental irritants.
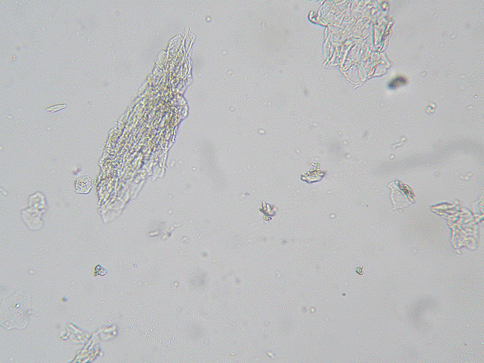
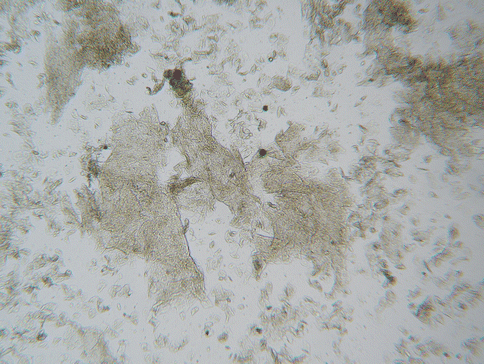

Fig. 19.1
High-power view of small skin flakes in an asymptomatic vaginal saline wet preparation

Fig. 19.2
Low-power view of numerous thick skin flakes in a vaginal saline wet preparation characteristic of symptomatic spongiotic dermatitis
More than 40 antimicrobial substances have been identified that are actively produced by the skin. These antimicrobial peptides are present and active in the stratum corneum [21]. For the vulva, these peptides likely have a significant role in the prevention of infection from fecal contamination. Human beta defensin-2 and human beta defensin-3 are antimicrobial peptides in this category that demonstrate significant anti-yeast inhibitory action [22]. The production of these antimicrobial peptides is linked with the normal cell-mediated immune response. Thus, a compromise of cell-mediated immunity can contribute to increased skin colonization with yeast, related to a deficiency of these important skin peptides. As would be expected, there is downregulation of human beta defensin-2 and human beta defensin-3 in spongiotic dermatitis [23]. This decline is a likely cofactor for the development of a genital yeast infection for women with vulvar spongiotic dermatitis. Similarly, a strong association between Th2 cytokines in the vulvovaginal area and a risk for yeast infection has been well documented [24]. A rise in estrogen increases the rate of allergy in women after puberty and, to a greater extent, during pregnancy [25]. Histamine release associated with allergy can cause prostaglandin E2 production which downregulates the local Th1 response, increasing the risk for yeast infection [24, 26]. Allergic dermatitis further inhibits Langerhans cell Th1 function. The clustering of Langerhans cells in spongiotic dermatitis that causes the characteristic microvesicles reflects a shift of vulvar epithelial Langerhans cells away from Th1 response. This decline in Th1 response significantly increases the risk for vulvovaginal yeast infection. Thus, just as all women are likely to experience vulvar spongiotic dermatitis at least once, all women should expect to have a vulvovaginal yeast infection at least occasionally. Some women suffer constantly from vulvar spongiotic dermatitis and remain at constant risk for yeast infection. Factors related to spongiotic dermatitis are possibly the most frequent cause for recurrent vulvar yeast or bacterial infection.
Chronic epithelial inflammation can result in an increased population of epithelial nerve endings and elongated nerve endings, likely resulting in progressively increasing skin sensitivity. This increase in nerve sensitivity has been linked to neurotropic substances that are activated as part of the normal inflammatory response. Nerve growth factor has a well-studied role in this regard [27]. This progressive increase in elongated epithelial nerve endings has been described in spongiotic dermatitis [28]. Similarly, gynecologic research has shown increased nerve endings in biopsy samples from cases of vulvodynia and vestibulitis [29, 30]. Unfortunately, the gynecologic samples were not investigated for spongiotic change in these reported nerve sensitivity studies. It is highly likely that chronic vulvar spongiotic dermatitis progressively increases nerve ending-mediated vulvar skin sensitivity.
19.6 Vulvar Sensitivity Changes After Menopause
After menopause, the vulva remains vulnerable to rubbing, with a persisting high friction coefficient [13]. For the postmenopausal vulva, estrogen deficiency and chronological aging are the main detrimental factors. The associated decline in barrier function and the loss of immune reactivity combine to influence the sensitivity of postmenopausal vulvar skin. The decline in barrier function provides a new opportunity for irritants to harm the increasingly fragile epithelium. The concurrent loss of immune reactivity may decrease the ability of the vulvar epithelium to register a symptomatic reaction. Thus, the relative awareness of vulvar sensitivity after menopause results from the interplay of multiple predictable factors that will be somewhat unique to the individual. With the lack of estrogen stimulation, vulvar skin would be expected to heal and recover more slowly after any insult.
The influence of aging upon skin is well documented [5, 6]. With aging, there is a significant decline in lipid production. Epidermal cells produce the lipids that fill the intercellular space. Lipid production consists of a fixed ratio of cholesterol, ceramides, and free fatty acids. Both chronological aging and loss of estrogen play a role in the loss of intercellular lipids. The age-related loss of the normal calcium gradient in the skin is one factor that adversely influences lipid production. The normal mixture of polar and nonpolar lipids in the stratum corneum contributes significantly to the epidermal barrier [31]. The normal composition of intercellular lipids is important for normal skin hydration. The degree of estrogen-associated keratinization of the stratum corneum is also important to maintain normal skin hydration. With aging and estrogen deficiency, intercellular lipid production is not adequately upregulated after injury. Yet, this significant defect in postmenopausal vulvar stratum corneum composition may still remain asymptomatic in the absence of mechanical or chemical damage. Considering the persistent exposure to environmental irritants, the potential for postmenopausal vulvar skin barrier problems may be greater than other body sites.
Estrogen loss and chronological aging lead to an adverse rise in vulvar skin pH after menopause [5, 6]. The normal skin pH of 4–5.5 maintains limited skin permeability, promotes skin cell cohesion, and regulates desquamation [32, 33]. A rise in the vulvar skin pH adversely affects these elements of normal skin physiology. The integrity of vulvar epithelium is profoundly compromised by a rise in pH. The rise in pH is directly associated with deficient intercellular lipid content. Loss of intercellular lipids, disruption of cell cohesion, and abnormal desquamation facilitate entry of microbial pathogens as well as environmental irritants into the epithelium. Traditionally, some degree of antimicrobial action is attributed to the normally low stratum corneum pH. This surface antimicrobial action is lost with aging. This combination of defects results in a possibility of higher rates of vulvar epithelial microbe colonization or infection as well as a greater potential for irritant reaction.
Bone marrow–derived Langerhans cells are distributed unevenly throughout the body. The highest concentration of Langerhans cells in the female genital tract is found in normal vulvar skin [34]. The greatest prevalence of Langerhans cells in vulvar epithelium is found during the reproductive years [35]. With aging, there is a significant decline in Langerhans cell function, as well as a fall in Langerhans cell count by approximately 50 % [36]. With aging, there is a significant decline in cytokine response that is linked to the decline in Langerhans cell function [37]. Thus, Langerhans cell-mediated immune response can be significantly blunted in older persons.
There is a clinically important decline in cell-mediated immunity with aging. For women, estrogen deficiency plays a primary role in this immune shift. Prior to puberty, the rate of asthma is the same for young boys and girls [25]. After puberty, under the influence of onset of estrogen production, asthma and a tendency for allergy increase for young girls. This immune shift toward allergy is potentiated with the further rise in estrogen levels during pregnancy. Then, after menopause, there is a decline in asthma and allergic response in older women [25]. The tendency for asthma is restored with hormone replacement therapy after menopause [38]. Thus, the ability of the vulvar immune system to produce a spongiotic reaction to environmental allergens is blunted after menopause. After menopause, dermatologic conditions such as lichen sclerosus would be expected to persist, but there is a decline in relative prevalence of vulvar spongiotic dermatitis. There is also a lower rate of allergy-associated vulvovaginal yeast infection unless immune responsiveness is restored by topical application of estrogen. After menopause, the rate of vulvovaginal yeast infection is significantly lower [39].
Early-stage vulvar squamous cell carcinoma can be misinterpreted as sensitive skin or vulvodynia. Langerhans cells also have a significant role in cancer surveillance. The postmenopausal risk for vulvar carcinoma increases with the decline in Langerhans cell function. Defense against squamous cancer-associated human papillomavirus rests in the phagocytic action of Langerhans cells. Half of vulvar carcinomas are attributed to HPV infection, and half relate alone to poor malignant cell surveillance by Langerhans cells [40].
In summary, the final status of relative vulvar sensitivity after menopause reflects an interplay of many independent factors. Atrophic vulvovaginitis may be the most universal risk for vulvovaginal burning after menopause. Postmenopausal barrier compromise increases susceptibility to irritants, allergens, and microbes. Decreased skin reactivity, reflected by decline in cell-mediated immunity, lowers the chance for symptomatic vulvitis. The rate of yeast infection is lower after menopause, unless estrogen is restored to the vulvar epithelium. If vulvar vestibulitis and vulvodynia are viewed as surrogates for vulvar sensitive skin syndrome, it is not surprising that surveys of postmenopausal women have not revealed a significant rise in vulvodynia after menopause [41, 42]. Interestingly, in these vulvodynia studies, the populations reporting vulvar symptoms differ before and after menopause. In contrast, a sensitive skin survey reported an increasing rate of self-reported genital skin sensitivity with aging, with little or no corresponding increase at other body sites [9]. Approximately 70 % of postmenopausal women reported somewhat sensitive genital skin, contrasting with 50–60 % of premenopausal women. Hot weather and abrasive clothing were the factors most significantly associated with reported sensitive skin after menopause. Others have actually reported a general decrease in skin sensitivity with age [43]. Thus, menopausal effects on skin sensitivity must be multifactorial with several concurrent changes where there may be actually elements that compensate for developing defects. Vulvar sensitivity factors prior to menopause differ somewhat from sensitive skin factors after menopause. Which women with sensitive skin prior to menopause continue to suffer after menopause depends upon which factors dominate. Vulvar sensitivity to mechanical abrasion may decline after menopause, but can recur after estrogen replacement therapy [44]. The barrier compromise due to lichen sclerosus is likely to persist. If spongiotic dermatitis is the prominent factor, then menopause may provide some relief unless topical estrogen is applied. Postmenopausal estrogen deficiency alone introduces elements of barrier compromise that increase susceptibility, especially to hydrophilic irritants. See Table 19.2.
Table 19.2




Factors that increase or decrease the tendency for vulvar skin sensitivity after menopause
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree





