Immediate Two-Stage Breast Reconstruction Using A Tissue Expander and Implant
Louis L. Strock
In recent years, tissue expansion has continued to grow in popularity as the preferred approach to immediate breast reconstruction. The appeal of immediate two-stage tissue expander with implant breast reconstruction lies in its relative simplicity, lack of donor-site recovery, and quicker return to normal activities as compared with autologous tissue breast reconstruction (1). The traditional approaches to autologous tissue immediate breast reconstruction, including transverse rectus abdominis rectus (TRAM) flaps and latissimus dorsi myocutaneous flaps, are reliable and remain popular but lead to significant donor morbidity that has become less accepted by many patients and surgeons as the results with the tissue expander implant approaches have improved. While some of these issues relating to donor area recovery have been addressed by the recent refinements in perforator flap techniques, these flaps have limited appeal due to the need for microsurgical expertise and long operative times. Additional issues that have contributed to the increase in relative importance of tissue expander and implant reconstruction include changes in breast cancer treatment, improvements in tissue expander devices, availability of silicone gel breast implants, and refinements in surgical technique in two-stage tissue expander implant reconstruction.
The treatment of breast cancer has shown numerous changes in recent years, starting with the trend toward aggressive application of immediate breast reconstruction using skin-sparing mastectomy techniques. The addition of skin-sparing techniques, to the extent permitted by skin involvement with oncologic issues, has been a major factor in contributing to the progressive improvement in results of immediate breast reconstruction, whether with autologous tissue or a tissue expander with implant approach. As results with immediate reconstruction have progressively improved, oncologic surgeons have accepted these approaches, and patients have also become more insistent that an attempt be made to reconstruct the breast mound at the time of mastectomy with preservation of as much of the native breast skin as possible. Additional advantages of this approach for patients include the ability to combine the recoveries of the reconstruction procedure with that of the mastectomy, combined with the benefit of speeding the time course of the reconstruction process, often with a more appealing aesthetic appearance during the tissue expansion process. Experience has shown that it is difficult to accomplish a result with delayed reconstruction that is comparable to that possible with immediate tissue expander with implant reconstruction using a skin-sparing technique at the time of mastectomy.
Another important recent trend is an increase in the popularity of prophylactic mastectomy as a contralateral procedure in patients with a confirmed diagnosis of breast cancer or as a bilateral procedure due to an increased risk of a breast cancer diagnosis. This has resulted in an increase in the number of patients who request bilateral mastectomy reconstruction (2). Many of these patients are slender and do not have adequate donor tissue to allow for bilateral autologous tissue reconstruction. In addition, some of these patients have had previous breast augmentation, making them more likely to pursue implant-based breast reconstruction. Recent experience also suggests that, as results improve with immediate tissue expander and implant reconstruction in this patient population, the appeal of this approach will likely increase further in the future.
In the years since breast reconstruction using tissue expansion began with Radovan’s description of a two-stage process that used a smooth, round tissue expander device, there has been a steady trend toward textured surface devices with anatomic shapes (3,4,5) (Figs. 32.1 and 32.2). Most recently these devices have been designed to provide choices, with a variety of device heights and projections that can be chosen according to the needs of each patient and reconstructive surgeon. Surgeons tend to develop a preference for a particular device height, as reflected in the recent literature on series by Cordiero and McCarthy (6), Spear and Pelletiere (2), and Strock (1), who favor full-height, mid-height, and low-height tissue expander devices, respectively. Review of these reports suggests that device height may not as important an issue in terms of outcome of two-stage expander implant breast reconstruction as compared with the combined use of integrated valve devices, placement in a submuscular or partial submuscular plane, and tissue release to the subcutaneous plane at the level of the inframammary fold, whether at initial expander placement or at second-stage permanent implant placement (1).
The importance of the reintroduction of silicone gel devices in the second stage of two-stage tissue expander cannot be overstated, because of the dramatic improvements in aesthetic appearance and softness compared with earlier limitations posed by saline permanent implant use in breast reconstruction. Current options include choices of round or anatomic shaped implants, smooth or textured surfaces, and silicone gel or saline device filler materials. The silicone gel implant devices are also further subdivided according to the level of cohesive properties of the silicone gel inherent in the device, with resultant differences in implant softness and potential for gel migration in the event of rupture. The use of silicone gel breast implants in tissue expander with implant breast reconstruction has also resulted in a marked decrease in the need for latissimus dorsi myocutaneous flaps, used for many years to provide added tissue cover to hide the flaws and limitations of saline breast implants in this context.
The recent availability of acellular dermal matrix materials has also provided a means to add tissue cover for expander implant breast reconstruction without resorting to the use of muscle flaps previously used routinely for this purpose. Recent reports suggest that the use of these materials can enhance the result of two-stage tissue expander with implant breast reconstruction by
distributing tension more evenly across the pectoral muscle, thus facilitating the control of shape along the inframammary fold (7). The added tissue cover helps to minimize visible rippling and other consequences of otherwise thin tissue cover (8). Additional experience will likely show that this approach will continue to grow in popularity, in its ability to both enhance the control of shape and reduce morbidity in two-stage expander implant breast reconstruction.
distributing tension more evenly across the pectoral muscle, thus facilitating the control of shape along the inframammary fold (7). The added tissue cover helps to minimize visible rippling and other consequences of otherwise thin tissue cover (8). Additional experience will likely show that this approach will continue to grow in popularity, in its ability to both enhance the control of shape and reduce morbidity in two-stage expander implant breast reconstruction.
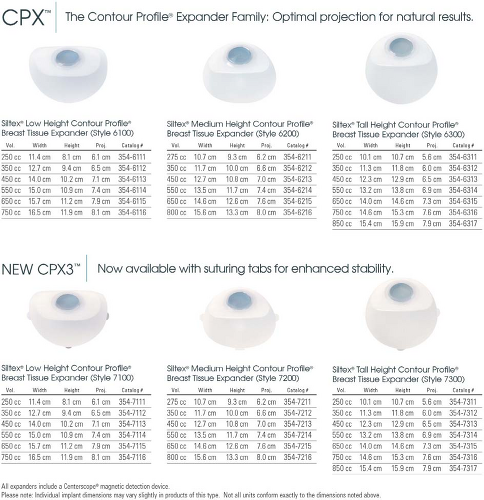 Figure 32.1. Tissue expanders available from Mentor Corp. (Santa Barbara, CA), with choices of height and projection. |
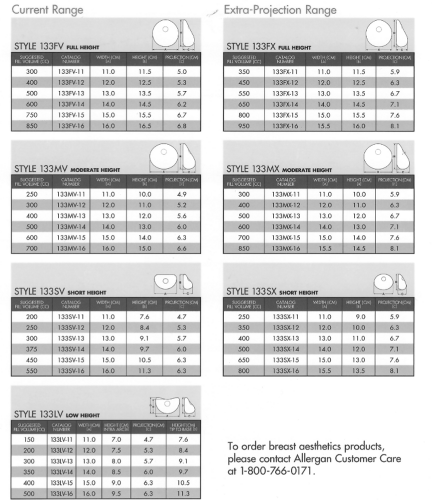 Figure 32.2. Tissue expanders available from Allergan Corp. (Goleta, CA), with choices of height and projection. |
An additional issue that merits mention is the question of whether the tissue expansion phase of this two-stage immediate tissue expander and implant approach is needed at all (9). It can be tempting in certain patients to move to immediate implant reconstruction, seemingly “skipping a step.” There are relatively few patients in whom this approach is helpful, because of persistent distortions of shape that result from the differential laxity of an abundant amount of skin compared with the invariably tight deeper tissue pocket and resultant distortion of the shape of the permanent implant. Some of these issues can be addressed by addition of large pieces of acellular dermal matrix materials placed in a fashion similar to the inset of a latissimus dorsi muscle flap as a loose soft tissue sling covering the implant in the subcutaneous plane. My experience, however, has consistently resulted in the need for a refinement procedure that calls into question not using a tissue expander aggressively filled at the time of mastectomy that has the advantage of volume adjustment as needed. I suggest caution in creating an expectation that a “second-step” procedure can be consistently avoided, as such disappointments are particularly difficult and frustrating for patients experiencing chemotherapy or additional adjuvant treatment for breast cancer following mastectomy.
Immediate two-stage tissue expander with implant breast reconstruction is not simple to perform. While this approach is advantageous for many patients for many of the reasons just listed, many challenges remain. The process is one in which precise control over thin tissue components must be carefully considered and managed at all times. Optimal aesthetic and functional results demand tremendous attention to detail, both in planning and technique, and a constant drive for improvement. The remainder of this chapter will attempt to highlight those issues in planning and technique that have helped to create consistent and reliable results, discussed with the understanding that all can be improved over time.
Patient Selection
Success in immediate two-stage tissue expander with implant breast reconstruction begins with careful patient selection. This begins with an assessment of the patient’s relevant medical issues and issues relating to her cancer treatment. This assessment usually must assume that the patient will need adjuvant chemotherapy, which will typically begin somewhere between 4 and 6 weeks following mastectomy. While the expectation would be that most patients would be able to heal from mastectomy and tissue expander placement by that time, patients with certain medical problems or history of severe tobacco abuse may not be appropriate candidates for immediate reconstruction. Relative to additional cancer treatment, any possible need for radiation must be carefully evaluated and discussed with the patient and her oncologic surgeon, medical oncologist, and radiation oncologist. If radiation treatment is planned to the mastectomy site, a collective decision must be made as to whether to proceed with immediate two-stage tissue expander and implant reconstruction. In selected patients, immediate two-stage tissue expander and implant reconstruction can be safely performed if the tissue expansion process is completed prior to the start of radiation therapy. Alternatively, the more common approach would be to defer immediate reconstruction and proceed with delayed reconstruction using autologous tissue following completion of all adjuvant treatment. In the case of a patient who has undergone previous adjuvant radiation to the breast being reconstructed, I favor the addition of nonradiated autologous tissue to provide a tissue interface between the tissue expander and the radiated tissue to be expanded.
The unilateral mastectomy patient is approached in a similar fashion. Immediate two-stage tissue expander and implant reconstruction is preferred in the slender patient, as these patients will rarely have adequate donor tissue available for autologous tissue techniques. Size goals in these patients will often lead to a plan that will require that an implant be needed for symmetry on the contralateral breast. The issues in the heavier patient having unilateral reconstruction are somewhat different because these patients may be ideally suited for some autologous tissue approaches because of the amount of donor tissue available. A TRAM flap or abdominal perforator flap is an excellent approach in a slightly heavier patient who tends to have an adequate soft tissue donor area for unilateral reconstruction. Obese patients tend to not be ideal candidates for any approach to immediate breast reconstruction, although they may be appropriate candidates, and are considered on an individual basis, depending upon motivational and medical assessment.
Any discussion of patients ideally suited for immediate two-stage tissue expander with implant breast reconstruction starts with slender patients who have a small to medium breast size and seek bilateral reconstruction. These patients are usually not candidates for lumpectomy or breast conservation because of the deformity that would result, leading these patients to mastectomy. This type of patient usually does not have adequate tissue for autologous reconstruction to be an appealing option, unless they have very limited size goals and are willing to endure the recovery associated with autologous tissue reconstruction. As the patient’s breast size goals increase, the immediate two-stage tissue expander with implant approach is preferred, with tissue expansion needed to create space adequate for any permanent implant to be used. Many of these patients seek a larger breast size and have had implants previously or would like to have had implants previously, which makes implant-based reconstruction even more appealing. As we consider heavier patients who seek immediate bilateral reconstruction, increasing levels of difficulty are encountered with all types of reconstruction, whether implant based or autologous. Many of these patients elect to pursue two-stage tissue expander with implant reconstruction to avoid the recovery associated with bilateral autologous tissue reconstruction in the heavy patient, a journey that can be even more challenging than that with expander implant reconstruction. Acellular dermal matrix materials are particularly helpful in this group of patients to help provide added tissue cover for a loose tissue sling made in combination with the pectoralis major muscle, placed over a tissue expander that is filled to project to the degree that the skin is lax. This can help these patients to have a quicker course of tissue expansion.
Planning
Once immediate two-stage expander and implant breast reconstruction has been chosen as the planned approach for reconstruction, specific operative planning is performed. The skin pattern for mastectomy is coordinated with the oncologic surgeon and discussed with the patient, with the understanding that most mastectomy scarring is difficult to see by 18 months postoperatively (Fig. 32.3). The most important component to the planning process is a measurement of the base width of the breast (Fig. 32.4). This is the basis for the biodimensional approach to breast reconstruction, and is compared with what would appear to be the ideal width of the planned reconstructed breast. The differences between these measurements can be important. For example, if the patient has a very small breast size and the goal is to reconstruct her breast to a larger size that more optimally fits her chest, a tissue expander that is slightly wider than the preoperative breast width is selected. For unilateral reconstruction, the correct expander width selection ultimately depends upon the planned width of the contralateral breast, whether it is to be modified or not. If the patient has large breasts and is overweight, by contrast, the breast width measurement may significantly exceed the width of the patient’s ribcage and must be accounted for in ultimate tissue expander size selection. These issues must be anticipated preoperatively and accounted for by having multiple choices of possible device widths available, pending intraoperative findings at the time of mastectomy.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








