ADM
Year introduced
Supplier
Location
Material
Cross-linked
Sterilized (method)
Lyophilized
Hydration/soak time
Refrigeration required
Shelf life (years)
AlloDerm
1994a
LifeCell
Branchburg, NJ, SA
Human dermis
No
No (aseptically processed)
Yes
10–40 min (2 steps)
Yes
2a
AlloMax
Davol (CR Bard) (processed by RTI Biologics)
Warwick, RI,USA
Human dermis
No
Yes (gamma irradiation)
No (supplied dehydrated)2
‘Rapidly’
No
5
DermaMatrix
2005a
Synthes CMF (processed by MTF)
West Chester, PA, USA
Human dermis
No
No (aseptically processed)
Yes
3 min
No
3
DermaSpan
2011b
Biomet
Warsaw, IN, USA
Human dermis
Nob
Yes (gamma irradiation)
Yes
15–45 min
No
2
FlexHD
2007a
Ethicon (J&J) (processed by MTF)
Human dermis
Nob
No (aseptically processed)a
No
None
Noa
3
Repriza
2010b
Specialty Surgical Products
Victor, MT, USA
Human dermis
No
Yes (irradiation)
No
None
No
2
Table 24.2
Comparison and summary of xenograft ADM
ADM | Year introduced | Supplier | Location | Material | Cross-linked | Sterilized (method) | Lyophilized | Hydration/soak time | Refrigeration required | Shelf life (years) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Permacol | 2000b | Covidien | Norwalk, CT, USA | Porcine dermis | Yes (HMDI) | Yes (gamma irradiation) | No (supplied moist) | None | No | 3 | |||||
Strattice | 2008a | Lifecell | Branchburg, NJ, USA | Porcine dermis | No | Yes | No (supplied moist) | ≥2 min | No | 2a | |||||
SurgiMend | 2006 | TEI Biosciences | Boston, MA, USA | Bovine dermis | No | Yes (ethylene oxide) | Yes | 60 s | No | 3 | |||||
Veritas | 2001 | Synovis | St. Paul, MN, USA | Bovine pericardium | No, propylene oxide capped amine technology | Yes (irradiation) | No | None | No | 2a | |||||
XenMatrix | 2006 | Davol (CR Bard) | Warwick, RI, USA | Porcine dermis | No | Yes | No | None | No | 5 | |||||
Immune reactivity, i.e. host adoption without inflammation
Handling qualities
Structural support ability
Collagen matrix properties (no chemical cross-linking)
Tissue incorporation and integration ability
Tissue regeneration ability
Cell revascularization ability.
ADMs are sourced from allogenic human cadaveric/bariatric dermis or from xenogenic tissues (porcine or bovine; dermis, pericardium, intestinal submucosa). They differ in thickness from less than 1 mm to over 4 mm, with the latter best suited for cosmetic purposes, where bulking is desired, or for large ventral abdominal hernias, where strength is desired.
Whereas human ADMs typically come in various rectangular sizes, some xenogenic ADMs are provided in shapes more suited to the subsequent three-dimensional conformation a flat sheet will take when placed over an implant. Such shaping, as well as premade fenestrations, helps the ADM to conform to the implant without pleating or wrinkling.
Human cadaveric ADMs typically maintain greater intraoerative and postoperative stretch than do xenogenic ADMs. Care and thought must be given in anticipating the potential for gradual ‘window-shading’ of the ADM higher on the upper pole of an expander during filling when a human ADM is employed. When using a less extensible xenogenic ADM, one must anticipate further travel of the inferior margin of the pectoralis major muscle towards the IMF during expansion.
24.4 Technique and Surgical Considerations
24.4.1 The Perfect Skin-Sparing Mastectomy
The perfect breast reconstruction depends upon the perfect mastectomy. Although the planning, decision-making and technical execution of the reconstructive component are important, many of the short-term and long-term complications from immediate reconstruction are mostly related to a suboptimal mastectomy.
24.4.1.1 Who Should Perform the Mastectomy?
It is not important whether a general or a plastic surgeon performs the mastectomy, provided the surgeon has the appropriate training and skills to be able to safely find and then stay within the mastectomy plane.
24.4.1.2 Where Is the Mastectomy Plane?
The mastectomy plane lies between the subcutaneous fat and the superficial fascia of the breast, crossed by the ligaments of Cooper that travel through the subcutaneous fat to anchor in the dermis (Fig. 24.1a). There is a conventional view that the superficial fascial plane is not reliably present and thus the plane may not always be identifiable. This appears to be based on an often-quoted small observational study [10] of breast-reduction specimens. There is, however, a compelling embryological explanation for the constant presence of this fascia, even if patient factors (extremes of BMI) or surgical factors (poor or closed techniques) mean that it is not always visualized. The superficial fascia is formed as a condensation from the sixth embryological week, when the primary ectodermal breast bud invaginates into the underlying mesenchyme [11].
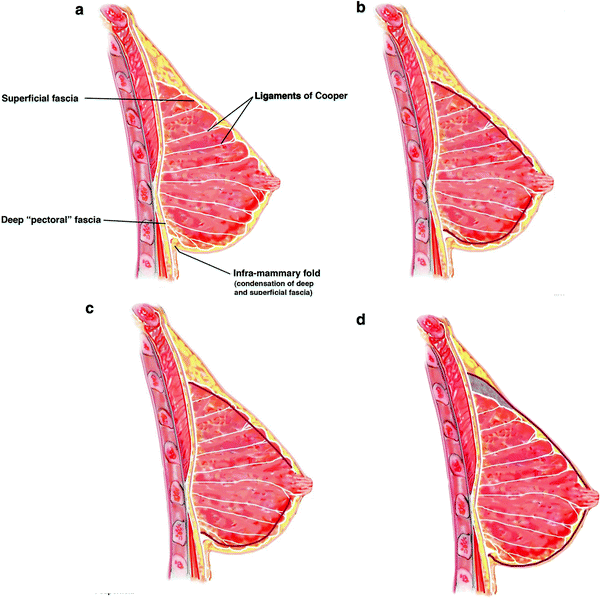

Fig. 24.1
Sagittal views of the breast demonstrating a fascial planes and ligamentous anatomy, b ‘thin’ skin flaps (increased risk of skin necrosis and unnecessary subcutaneous fat excision above the breast), c ‘thick’ skin flaps (increased risk of residual breast tissue and local recurrence) and d ‘ideal’ mastectomy plane over superficial fascia
Regardless of the technique and instruments used, achieving the correct dissection plane is essential for optimal oncological safety and viability of the skin envelope. A ‘thin’ or traumatized skin flap is more likely to have compromised perfusion. A ‘thick’ skin flap is more likely to carry residual breast tissue with an unnecessary increased risk of future disease or local recurrence (Fig. 24.1b, c). There are several well-designed studies that demonstrate residual breast tissue left on mastectomy skin flaps in up to 50 % of biopsies looked at [12–14]. Without evidence of intact superficial fascia on the mastectomy specimens removed, such studies should be interpreted with caution.
It should be remembered that the ‘ideal’ skin flap thickness is proportionate to a patients BMI and body habitus and is therefore ‘patient-dependent’ not ‘surgeon-dependent’. It should be possible to aim for complete removal of the breast tissue, and breast surgeons should continue to strive for the cleanest possible dissection in the plane; i.e. over the fascia, with division of the ligaments of Cooper as close to dermal attachments as possible (Fig. 24.1d).
24.4.1.3 What Is the Best Technique for Performing Skin-Sparing Mastectomy?
Planning the mastectomy must take into account the three-dimensional shape of the envelope, the likely tension on the skin and the access that the incision will give, for both the least traumatic removal of the gland and the safest, most accurate insertion of the implant.
Once the optimal amount of skin (with or without nipple) for the best envelope and reconstruction has been decided upon, the joint surgical objectives are:
To optimize oncological safety—removing all breast tissue whilst respecting the mastectomy plane and envelope landmarks
To optimize envelope viability—not compromising the perfusion of the skin envelope.
There is no agreement, nor need there be, on the single best technique for performing skin sparing mastectomy. Some surgeons find infiltration helpful to develop the plane (with or without adrenaline). Alternatively a dry technique with direct visualization of the fascia and ligaments may be preferred. Scalpel, scissors, diathermy electrodissection, ultrasound, laser and argon all have their advocates. In selecting the technique for mastectomy, every surgeon must decide how best to reconcile the compromise among ease of dissection, speed, haemostasis and the development of complications such as seroma, haematoma and skin necrosis.
Finally, the appropriate selection of the technique and instruments to use for a specific mastectomy should be based not on a surgeon’s routine preference, but after consideration of that patient’s individual soft tissue characteristics and risk factors for skin necrosis (obesity, smoking status, etc.,).
24.4.2 Classification of Skin-Sparing Mastectomy with use of the ADM Technique
An algorithm for mastectomy and technique selection for implant-based reconstruction with lower pole support is show in Fig. 24.2.
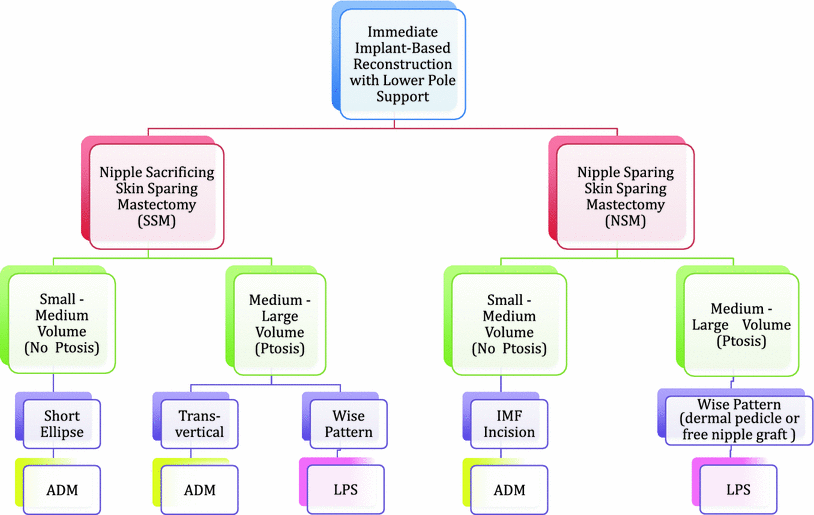

Fig. 24.2
Mastectomy and technique selection algorithm for implant-based reconstruction with lower pole support. ADM acellular dermal matrix, IMF inframammary fold, LPS lower pole derma sling
24.4.2.1 Skin-Sparing Mastectomy in the Non-ptotic Breast: The Short Ellipse Incision
When the nipple is to be sacrificed, our preference is for a short ellipse including the nipple with an oblique orientation (Fig. 24.3a). The dimensions and exact orientation of the ellipse should take into account the desired final three-dimensional shape and volume of the breast. The incision may require a short ‘lazy-S’ lateral extension, so that it is large enough to allow safe access for mastectomy, accurate insetting of the ADM and access to the axilla if necessary. Excess skin should be excised with caution and after consideration of the characteristics of the skin envelope (elasticity, compliance, possible perfusion problems) as well as how to achieve a comfortable fit between the implant domain and the skin envelope. It is always possible to modify and excise further if there is large skin excess when the envelope is redraped over the newly created mound. The oblique scar created is usually not conspicuous after nipple–areola reconstruction (Fig. 24.4).
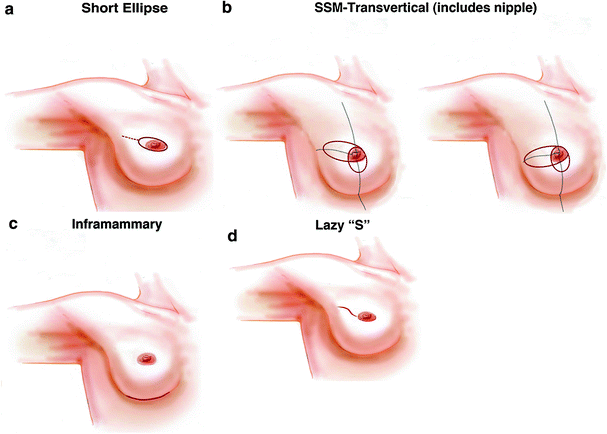
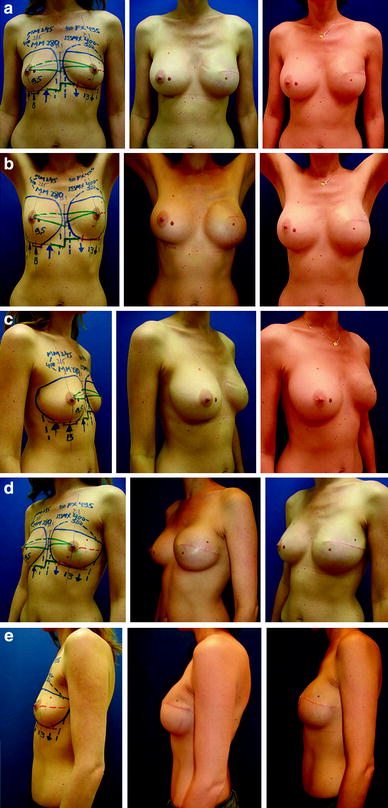

Fig. 24.3
Mastectomy incisions for use with the ADM technique: a short ellipse incision with or without ‘lazy-S’ lateral extension (skin sparing mastectomy); b transvertical incision (skin-reducing mastectomy); c inframammary incision (nipple-sparing mastectomy); d ‘lazy-S’ oblique lateral incision (nipple-sparing mastectomy)

Fig. 24.4
Left skin-sparing mastectomy (270 g) with short elliptical oblique incision and two-stage reconstruction with an expander and ADM (Natrelle Style 133 MX500, Surgimend 10 cm × 15 cm) and then a definitive implant (Natrelle Style 410 MX550). Contralateral right dual-plane augmentation in the first stage (Natrelle Style 410 MM280). Preoperative and postoperative images demonstrating intermediate and final outcome following refinement with fat-grafting
24.4.2.2 Skin-Reducing Mastectomy in the Large or Ptotic Breast: The Transvertical Incision
If an ADM is to be used rather than an LPS, then our preference is for the transvertical approach (Fig. 24.3b), which combines two vectored skin excisions—the larger, horizontal one is placed lateral or oblique to the nipple–areola complex (NAC) and the shorter, vertical elliptical excision overlaps the former in the NAC area. The resultant skin envelope has a more pleasing final shape and a better positioned scar than if a longer, wider oblique or transverse ellipse is used. The transvertical approach avoids the potential ischaemia-related wound-healing problems encountered by some surgeons when using the Wise-pattern skin envelope (Figs. 24.5 and 24.6).
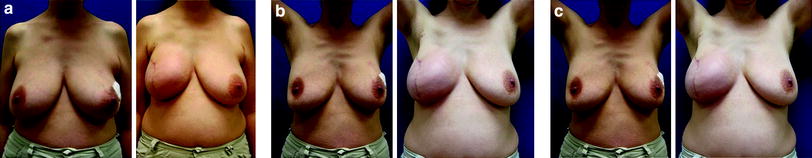
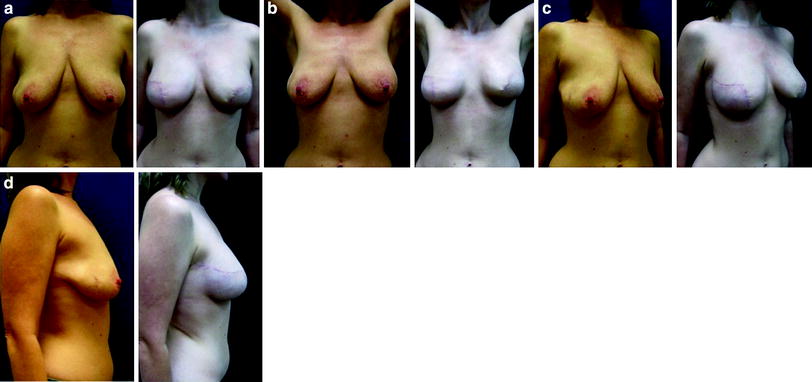

Fig. 24.5
Skin-reducing mastectomy with transvertical incision and immediate implant and ADM reconstruction (Natrelle Style 410 FX615, Surgimend 10 cm × 20 cm). Preoperative and postoperative images: a 42-year-old woman with multifocal carcinoma in the right breast (872 g)

Fig. 24.6
Bilateral skin-reducing mastectomy with transvertical incisions and immediate implant and ADM reconstructions (Natrelle Style 410 FF335, Surgimend 10 cm × 15 cm). Preoperative and postoperative images: a 47-year-old, BRCA1 gene carrier (right breast 295 g, left breast 315 g)
24.4.2.3 Nipple-Sparing Mastectomy in the Small to Moderate-Sized Breast: The Inframammary Incision
Traditional periareolar and circumareolar incisions have been shown in the best centres to have an increased risk of nipple–areola necrosis [15, 16]. Although it is possible to use an oblique orientated ‘lazy-S’ upper outer quadrant incision (Fig. 24.3d), our preference is for the use of an inframammary incision whenever possible (Fig. 24.3c). Although this is more technically challenging, there is less of a risk to nipple viability. The resultant access to the lower pole is ideal for the accurate insertion of the ADM and affords precise control and fixation of the IMF. It also produces a very favourable and ‘hidden’ scar (Figs. 24.7 and 24.8).
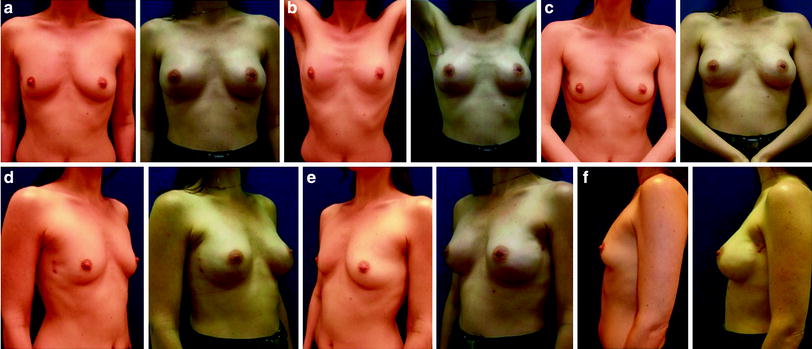
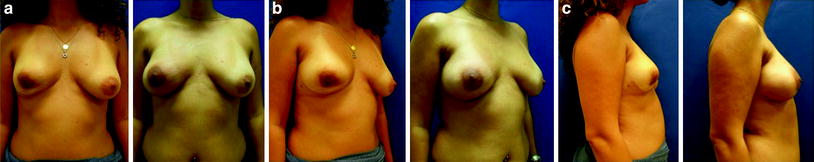

Fig. 24.7
Bilateral nipple-sparing mastectomy with inframammary incision and immediate implant and ADM reconstructions (Natrelle Style 410 MX325, Surgimend 10 cm × 15 cm). Preoperative and postoperative images: a 38-year-old, BRCA1 gene carrier with carcinoma in the right breast (120 g) and risk-reducing mastectomy of the left breast (133 g)

Fig. 24.8
Nipple-sparing mastectomy with inframammary incisions and immediate implant and ADM reconstructions (Natrelle Style 410 FX410, Surgimend 10 cm × 15 cm). Preoperative and postoperative images: a 35-year-old woman requiring complete right mastectomy (350 g) after incomplete excision of carcinoma (wide excision 75 g)
As mentioned earlier, the technique and instrumentation chosen for mastectomy through the IMF incision is less important than the surgeon’s ability to produce a healthy, non-traumatized skin envelope and a well-perfused nipple. Where access is difficult, the use of a headlight and delicate use of retractors is essential. Great care must be taken by the surgeon and assistant to avoid mechanical crush of the lower pole skin. An endoscope may be useful in the large breast (video-assisted mastectomy) for direct visualization of the medial, superior and lateral extent of the envelope, thus minimizing retraction injury or damage to the important skin perforator vessels.
Although the risk of occult nipple involvement or future nipple disease is acceptably low, provided predictive criteria for further nipple disease are followed [17, 18], we still recommend a subareolar ductal biopsy in all cases of nipple preservation with intraoperative frozen section. This requires close collaboration with an excellent histopathologist with a low false-negative rate for detecting occult disease on frozen section. Others may prefer to perform preoperative MRI, staged subareolar duct excision or subareolar vacuum-assisted biopsy prior to making a decision about the safety of nipple preservation. If the frozen section (or subsequent pathology report) demonstrates occult subareolar disease, then the nipple must be excised intraoperatively (or in a second procedure).
24.4.3 Classification of Skin-Reducing Mastectomy with the LPS Technique
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








