Hyaluronic Acid and Other Soft Tissue Fillers for Facial Rejuvenation
David R. Jordan
EDITOR COMMENTARY
The search for an ideal soft tissue filler to correct various facial folds and wrinkles is ongoing. This chapter highlights use of the soft tissue fillers (hyaluronic acid products, polymethylmethacrylate [PMMA] beads wrapped in collagen) that have become popular in many parts of the world. Both Drs. Jordan and Bosniak have extensive experience using these products.
Background
The search for an ideal soft tissue filler to correct various facial folds and wrinkles has been ongoing for years. At the turn of the last century, injectable paraffin was tried but quickly found to be unacceptable (1). A refined form of liquid silicone was introduced in the 1960s, and for the next 30 years various forms of injectable silicone were used. Unfortunately, numerous complications arose from the use of adulterated or impure silicones. As a result, injectable silicones were abandoned (2). Recently, however, a renewed interest in commercially available high-viscosity silicone products (Adatosil 5000, Silikon 1000) for soft tissue filling is taking place in Mexico and some centers in the United States (3, 4 and 5). These products reportedly work well for lip augmentation, nasolabial folds, and human immunodeficiency virus (HIV) lipodystrophy, but they are of little use for periocular wrinkles (3, 4 and 5). Autologous fat, another soft tissue filler, has been around for decades. In more recent years it has increased in popularity primarily due to improved harvesting techniques (6,7). The longevity of contour correction following autologous fat is somewhat variable and appears to be dependent on several factors, including techniques used, amount of fat injected, location of recipient site, and type of defect being treated.
In the early 1980s, after extensive trials, injectable bovine collagen was introduced (Zyderm, Zyplast) and has become a gold standard against which other newly introduced injectables are assessed (8). They are easily administered, safe,
and effective in short-term soft tissue augmentation. However, the possibility of allergic reactions to bovine collagen and the limited duration of effect have stimulated the continued research for more ideal fillers (9). In more recent years, other collagen products have been introduced. Autologous injectable human collagen (Autologen, Collagenesis Inc., Beverly, MA) was processed from surgically excised skin (10). Dermalogen, an injectable human collagen manufactured from cadaver skin, was available from the same company and was significantly less costly. Unfortunately, Collagenesis filed for bankruptcy in September 2001 and the product became unavailable. Dermologen, however, may soon be available again (Collagen Matrix Technologies, Inc.; S. Fagien, personal communication, 2003). Isologen (Isologen Inc., Houston, TX) is a living system of cultured and expanded autologous fibroblasts. Tissue from a non-sun-exposed skin area is obtained using a 3-mm punch, and the collagen is harvested and grown in culture. The process is labor intensive and time consuming, and it requires that the patient wait for the tissue to be grown (11,12). At the time of writing this book, Isologen is undergoing United States Food and Drug Administration (FDA) trials. CosmoDerm (for fine lines) and CosmoPlast (for deeper wrinkles and folds) (Inamed Aesthetics, Santa Barbara, CA) are the most recent collagen replacement tissue filler to appear on the market (United States and Canada). They contain human collagen purified from dermal tissue and grown under controlled laboratory conditions. A pretreatment skin test is not required prior to injection. Both are FDA approved (March 2003). Other tissue fillers popularized in recent years throughout Europe and now in Canada include injectable hyaluronic acid derivatives (Restylane, Hylaform, Juvéderm), dextran beads suspended in hyaluronic acid (Reviderm), and PMMA microspheres suspended in bovine collagen (Artecoll).
and effective in short-term soft tissue augmentation. However, the possibility of allergic reactions to bovine collagen and the limited duration of effect have stimulated the continued research for more ideal fillers (9). In more recent years, other collagen products have been introduced. Autologous injectable human collagen (Autologen, Collagenesis Inc., Beverly, MA) was processed from surgically excised skin (10). Dermalogen, an injectable human collagen manufactured from cadaver skin, was available from the same company and was significantly less costly. Unfortunately, Collagenesis filed for bankruptcy in September 2001 and the product became unavailable. Dermologen, however, may soon be available again (Collagen Matrix Technologies, Inc.; S. Fagien, personal communication, 2003). Isologen (Isologen Inc., Houston, TX) is a living system of cultured and expanded autologous fibroblasts. Tissue from a non-sun-exposed skin area is obtained using a 3-mm punch, and the collagen is harvested and grown in culture. The process is labor intensive and time consuming, and it requires that the patient wait for the tissue to be grown (11,12). At the time of writing this book, Isologen is undergoing United States Food and Drug Administration (FDA) trials. CosmoDerm (for fine lines) and CosmoPlast (for deeper wrinkles and folds) (Inamed Aesthetics, Santa Barbara, CA) are the most recent collagen replacement tissue filler to appear on the market (United States and Canada). They contain human collagen purified from dermal tissue and grown under controlled laboratory conditions. A pretreatment skin test is not required prior to injection. Both are FDA approved (March 2003). Other tissue fillers popularized in recent years throughout Europe and now in Canada include injectable hyaluronic acid derivatives (Restylane, Hylaform, Juvéderm), dextran beads suspended in hyaluronic acid (Reviderm), and PMMA microspheres suspended in bovine collagen (Artecoll).
Hyaluronic Derivatives
Hyaluronic acid is a naturally occurring polysaccharide that is a major component of all connective tissue. It exhibits no species or tissue specificity; the chemical structure of this polysaccharide is uniform throughout nature. There is no potential for immunologic reactions to hyaluronic acid in humans. The primary biologic function of hyaluronic acid is to provide stabilization to the intercellular structures and to form the elastoviscous fluid matrix in which collagen and elastic fibers become embedded. In addition, it regulates the transport of solutes in the extracellular space, regulates cell movement and functions, and plays a role in developing and remodeling tissue (13,14). In the skin, hyaluronic acid molecules bind water and create volume. The amount of hyaluronic acid in the skin decreases with age, and its loss results in reduced dermal hydration and increased skin wrinkling.
In its natural form, hyaluronic acid will be degraded very quickly by the body’s own enzymatic system. In order to obtain a volume-augmenting effect in the tissue over a longer period of time the, hyaluronic acid has to be modified slightly. By cross-linking hyaluronic acid through various patented processes, a number of companies have come up with hyaluronic acid products that exhibit a prolonged residence time in soft tissues and are biocompatible. The physical properties of hyaluronic acid gels are controlled by the molecular weight, the concentration of the material, and the degree of cross-linking. The most commonly used sources of hyaluronic acid are rooster combs, the umbilical cord, the vitreous humor, tendons, and bacterial cultures (14).
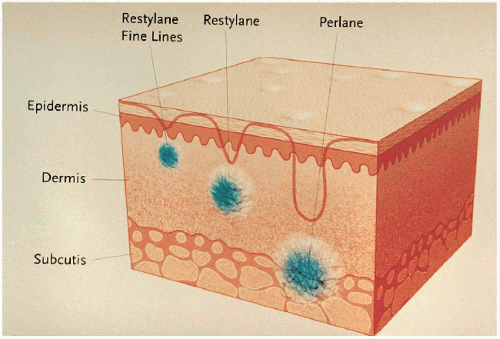
Restylane (Q-Med, Uppsala, Sweden) is a hyaluronic acid soft tissue filler that is made of hyaluronic acid biosynthetically produced through a bacterial fermentation process. The concentration of stabilized hyaluronic acid is 20 mg/mL. As of early 2003, three forms of the product are available (Restylane Fine Lines, Restylane,
Perlane), with a fourth on the horizon (Perlane Plus). Each is designed to be injected at different layers in the skin (Fig. 5.2B.1). The clinical difference between the products is the size of the gel particle. Restylane Fine Lines has 200,000 gel particles per milliliter and is used for fine superficial wrinkles. Restylane has 100,000 gel particles per milliliter and is used for larger wrinkles (glabellar furrows, nasolabial lines, lip augmentation) (Figs. 5.2B.2 and 5.2B.3). Perlane has 8,000 gel particles per milliliter and is used for deeper folds (nasolabial) and volume augmentation (lips) (Fig. 5.2B.4). Perlane Plus, the thickest product, has 4,000 gel particles per milliliter and will be used for deep folds and sculpting the cheek area.
These products are designed to last between 9 and 12 months. There is some individual variation, however, and in some patients it will last less than 9 to 12 months. Because there is no species or tissue specificity, there is little potential for immunologic reactions to the hyaluronic acid; therefore, skin testing is not required.
Perlane), with a fourth on the horizon (Perlane Plus). Each is designed to be injected at different layers in the skin (Fig. 5.2B.1). The clinical difference between the products is the size of the gel particle. Restylane Fine Lines has 200,000 gel particles per milliliter and is used for fine superficial wrinkles. Restylane has 100,000 gel particles per milliliter and is used for larger wrinkles (glabellar furrows, nasolabial lines, lip augmentation) (Figs. 5.2B.2 and 5.2B.3). Perlane has 8,000 gel particles per milliliter and is used for deeper folds (nasolabial) and volume augmentation (lips) (Fig. 5.2B.4). Perlane Plus, the thickest product, has 4,000 gel particles per milliliter and will be used for deep folds and sculpting the cheek area.
These products are designed to last between 9 and 12 months. There is some individual variation, however, and in some patients it will last less than 9 to 12 months. Because there is no species or tissue specificity, there is little potential for immunologic reactions to the hyaluronic acid; therefore, skin testing is not required.
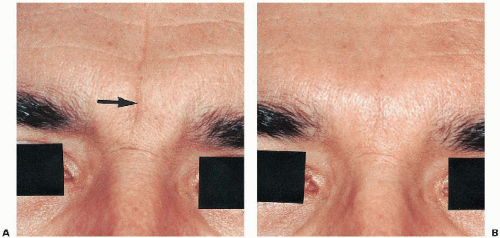 Figure 5.2B.2. A: Prominent vertical forehead line (arrow). Restylane (0.4 mL) was used. B: Four weeks after Restylane injection, with estimated 90% to 95% reduction of fold. |
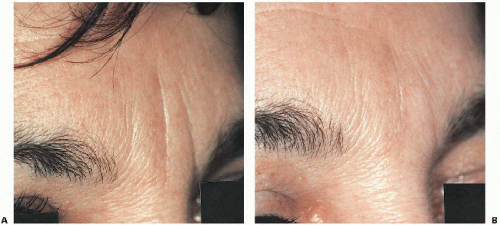 Figure 5.2B.3. A: Prominent vertical forehead lines. B: Eight weeks after Restylane injection, with estimated 80% to 85% reduction of folds. |
Hylaform (Biomatrix Inc., Ridgefield NJ, distributed in Canada by McGhan Medical, Toronto, Ontario) is another hyaluronic acid soft tissue filler similar to Restylane. The concentration of hyaluronic acid is 6 mg/mL and is extracted from rooster combs. Like the Restylane product line, Hylaform also is available in a thicker version (Hylaform Plus) and a thinner form (Hylaform FineLine). To date, there have been no reported instances of immunologic reactions to any residual avian products (13). Hylaform products are roughly equivalent to the Restylane products and used in similar anatomic areas (glabella, nasolabial folds, marionette lines, lip augmentation). Hylaform has a higher molecular weight, and its more viscoelastic properties have a more elastic tendency compared to the more viscous tendency of Restylane. The duration of effect with Hylaform may be slightly less than Restylane (6 months vs 9-12 months) but is individually dependent (product breakdown in faster in some individuals).
Rofilan is another hyaluronic acid derivative (Rofil Medical International, Breda, The Netherlands, distributed in Canada by Canderm Pharma Inc., St.-Laurent, Quebec) that, like Restylane, is manufactured from a bacterial fermentation process. The concentration of hyaluronic acid is 20 mg/mL. It was taken off the market in 2002 in Canada because its duration of action was far shorter (3 months) than expected.
Juvéderm is a recent export from France, where it has been used for the past few years. A product of L.E.A.Derm (info@leaderm.com), it is a hyaluronic acid that is of nonanimal origin. It is used in the same areas as the other hyaluronic products mentioned and comes in three versions: Juvéderm 18 for fine lines, Juvéderm 24 for mild-to-moderate wrinkles, and Juvéderm 30 for lips and deep folds and for sculpting the cheek area. The duration of action is 6 to 9 months but, like other hyaluronic acid products, is individually dependent.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree