Years
Development
1962
Implants in Sialastic® silicone gel—first generation
1965
Implants in Simaplast saline solution
1975
Implants in “low-bleed” silicone gel—second generation
1976
Implants with a double chamber
1976
Anatomic implants
1986
Implants coated in polyurethane
1988
Implants with a textured surface
1990
Implants with hydrogel
1992
Prohibition by the FDA of the use of silicone gel implants
1993
Implants with Trilucent lipid
1995
Anatomic implants in cohesive silicone gel—third generation
2002
Anatomic implants in silicone gel with differentiated shapes for the right and left breast (PIP implants)
2003
Implants coated in a titanium microstructure
2011
PIP and Rofill implant crises
20.2 Types of Implants
There are various types of breast implants and they can be categorized according to the characteristics of the material or the indications
Smooth, textured, polyurethane, and microtextured in titanium envelopes
Saline-filled, regular, or cohesive silicone gel, mixed gel and other nonhomologous substances (soybean oil, peanut oil, hydrogel, etc.)
Round and anatomic shapes or other shapes
Fixed or variable volume.
20.2.1 Saline Implants
These implants are made with a silicone envelope and a valve that allows inflation by means of a physiological solution during the surgical procedure. The envelope may be either smooth or textured. The envelope has good elasticity, and allows variation of the inflation from the saline solution in order to obtain better symmetry with the contralateral breast. Overinflation of 10–20 % more than the volume recommended by the implant manufacturer is suitable as it causes better distension of the envelope and prevents the implant folding. These folds could subsequently cause rupture of or extrusion of material from the prosthesis, especially in the case of thin and irradiated tissue coverage. The shape might be round or anatomic, but it is more difficult to maintain an anatomic shape when saline solution is used as the implant does not have the same consistency as cohesive gel. The valve may be anterior or posterior according to the technique. Small incisions can be adequate for insertion of the implant, and it can then be inflated with the physiological solution.
Practically, it would be simpler to use implants with anterior valves or periareolar and posterior valves for axillary incision cases. The valve is one of the critical points of saline implants, as a rare production defect may cause partial or total leakage of the physiological solution. Usually, this leakage does not produce any pathological damage to the patient, as it is a physiological solution and can be reabsorbed. The main problem is a good aesthetic result; the deflation can lead to the necessity for a revision or substitution procedure (Fig. 20.1). Some studies reported different levels of leakage or disruption of saline implants. A French group has demonstrated leakage level of 15 % in 650 patients with an average follow-up of 5 years [10].


Fig. 20.1
Round prostheses: physiological solution (with a valve for filling) and prosthesis in silicone gel
20.2.2 Silicone Gel Implants
These implants have a fixed volume. They are made of an envelope of silicone gel. Currently, the thickness of the envelope is carefully considered. It can be more resistant and avoid the “perspiration” of silicone gel particles. Silicone gel is elastomeric and its viscosity depends on the molecular mass, so now it is possible to manufacture a more cohesive gel. This kind of gel is used in the manufacture of anatomic implants, which need to be slightly more rigid in order to maintain the anatomic shape. Anatomic implants with cohesive gel were a great advance in terms of improving the aesthetic results in breast reconstruction. It is possible to achieve a better shape with less projection of the upper pole of the breast. Implant manufactures are now using three types of cohesive gel: low, medium and high cohesive gel for both anatomic and round implants. For this reason, plastic surgeons have a very large choice depending on the shape and consistency of the breast.
A technical refinement is necessary when using this implant for breast reconstruction. When the mastectomy flaps are thick and well vascularized, it is possible to make a partial cover of the implant with only the pectoralis major muscle. This technical element contributes to a better aesthetic result, with more projection of the lower pole of the breast. Using a round prosthesis with complete coverage of the pectoralis major muscle and the serratus anterior muscle may create a round and less natural shape [11]. Another advantage of cohesive gel is increasing patient safety by reducing the phenomenon of perspiration of the particles. As it is more cohesive, this gel probably reduces migration to the lymph nodes even in cases of disruption of implants. The main advantage of anatomic implants is the possibility to choose different shapes and volumes, as some manufacturers supply different models of implants that differ according to three parameters: height, width, and anterior projection. It is easier to select the ideal implant according to the different morphological characteristics of the patient (Fig. 20.2). Other manufacturers provide innovative features such as prostheses for the right or the left breast and with a concave posterior wall for thoracic wall convexity (Poly Implant Prosthèse, PIP, implant). Although this was an interesting idea, this manufacturer suffered a crisis in 2010 owing to suspicion of the use of industrial (nonmedical and nonapproved use in humans) silicone in their implants [12].
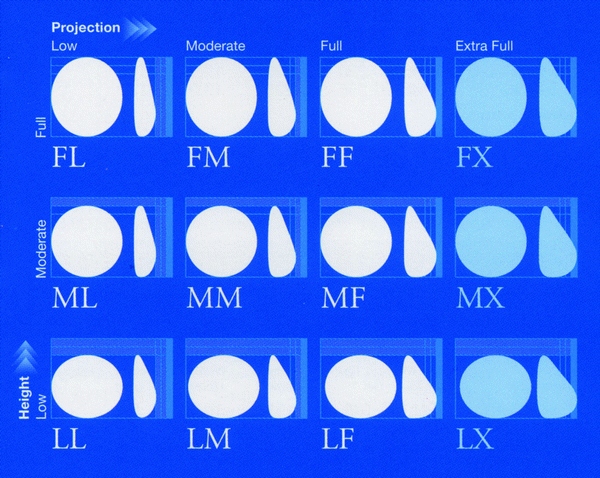

Fig. 20.2
Different models of anatomic prostheses following the parameters of base width, height, and projection
The disadvantages of anatomic implants with cohesive gel are:
Harder consistency of the reconstructed breast, which may create a periprosthetic capsular contracture.
Wider skin incision. In cosmetic augmentation mammaplasty, round implants can be inserted even through a small periareolar incision. In contrast, anatomic implants need bigger incisions, preferably in the inframammary fold, to allow compression-free insertion, as even a slight compression could deform them.
Increased risk of implant rotation. This is more frequent when there is a postoperative seroma or absence of periprosthetic capsular formation. Sometimes is possible to rerotate the implant into the correct position with a “manual massage”; if this is not possible, then a surgical revision is needed [13].
20.2.3 Double-Chamber Implants
These implants are made with an internal coat of silicone gel and an external chamber that can be filled with 20–50 ml of physiological solution. The initial purpose was to create a more fragile external chamber that will degrade 3 or 4 months after implantation, therefore reducing the implant volume by 20–50 cm3 when the periprosthetic capsule stabilizes. There was no clear advantage of reducing periprosthetic capsular contracture when compared with single-chamber implants, so the use of double-chamber prostheses was discontinued.
20.2.4 Polyurethane-Coated Implants
These implants are made with an external coat of polyurethane, and they are more efficient in preventing capsular contracture than those with a smooth envelope. The physical characteristic of the polyurethane-coated implant causes disorientation of the direction of collagen fibers, which does not occur in smooth-envelope implants [14]. Some publications have demonstrated that the metabolism of polyurethane produced 2,4-toluenediamine and 2,6-toluenediamine, which could be carcinogenic [7, 15]. The incidence of periprosthetic contracture and other complications was reported to be less than 1 % [16]. In the case of infection with a polyurethane prosthesis, removal of the prosthesis with all polyurethane residues is mandatory to avoid cutaneous fistula.
20.2.5 Titanium Microstructure Implants
These are relatively new implants, launched on the market in 2003, and they have the following characteristics: the internal part is silicone gel and the external envelope is silicone with a titanium microstructure. The aim is to reduce reactions to foreign bodies and consequently to decrease the incidence of periprosthetic capsules. An in vivo animal study show titanium-coated silicone grafts were not effective in protecting against infection; however, they might reduce the rate of capsular contracture [17].
20.2.6 Definitive Expanders
These implants have an adjustable volume. The external chamber is filled with silicone gel and an internal chamber can be filled with physiological solution; therefore, it is easier to match the volume symmetry of the opposite breast. The internal chamber lies at the lower portion of the implant; therefore it creates the anatomic-shape-like prosthesis. An external valve connects with the internal chamber through a 2-mm silicone tube; this device can be removed (Becker’s prosthesis) or fixed to the prosthesis (Allergan style 150). The valve can be easily placed in the axillary region or other superficial locations. A parasternal placement may cause discomfort to the patient and should be avoided [18]. This type of implant is also useful for an immediate breast reconstruction with a fragile skin flap and high risk of necrosis if high tension is found. In such cases, the implant can be inserted without filling the internal chamber, and inflation can be done after 3 or 4 weeks when a viable skin flap is approved. There is also the possibility to correct the volume when the body weight changes in the postoperative period owing to chemotherapy or hormone therapy (Figs. 20.3, 20.4).



Fig. 20.3
Anatomically differentiated prostheses, one shape for the right side and another shape for the left side

Fig. 20.4
Definitive expanders. These are prostheses with a silicone gel chamber and a second chamber with physiological solution, where the volume can be adjusted through a small subcutaneous valve. Prosthesis filled to the maximum level (left) and prosthesis without physiological solution filling (right)
Two disadvantages in the use of implants of this type are
1.
Patients experience some discomfort from an axillary valve. If the implant has a removable valve, it can be removed simultaneously with the nipple and areola complex reconstruction procedure. If the valve cannot be removed and the final volume is obtained, then it is possible to place the valve behind the implant when the subsequent surgical procedure is required.
2.
The point of connection of the tube on the implant surface is rather vulnerable. There is a risk of physiological solution leaking through the protection valve in a tube-removed implant. On other hand, there is a major mechanical traction force that may result in disruption of the implant if the tube is not removed.
20.2.7 Temporary Expanders
These are implants with an elastic silicone envelope and a filling valve that allows inflation by means of physiological solution. The postoperative skin can be expanded to match the volume symmetry of the contralateral skin. However, a second surgical procedure is needed to replace the expander with the definitive implant. There are different models and shapes of expanders: round or anatomic, with integrated valves or with separated valves. The older models are round and have separated valves which are connected with the device through a 2-mm-diameter silicone tube. The disadvantage of these models is that they not only produce distension of the lower pole but they also cause distension of the upper pole and the pectoralis major muscle. Such distension causes pain and discomfort when patients move their upper limbs; distension of the upper pole is more likely than distension of the lower pole (Fig. 20.5




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








