Introduction
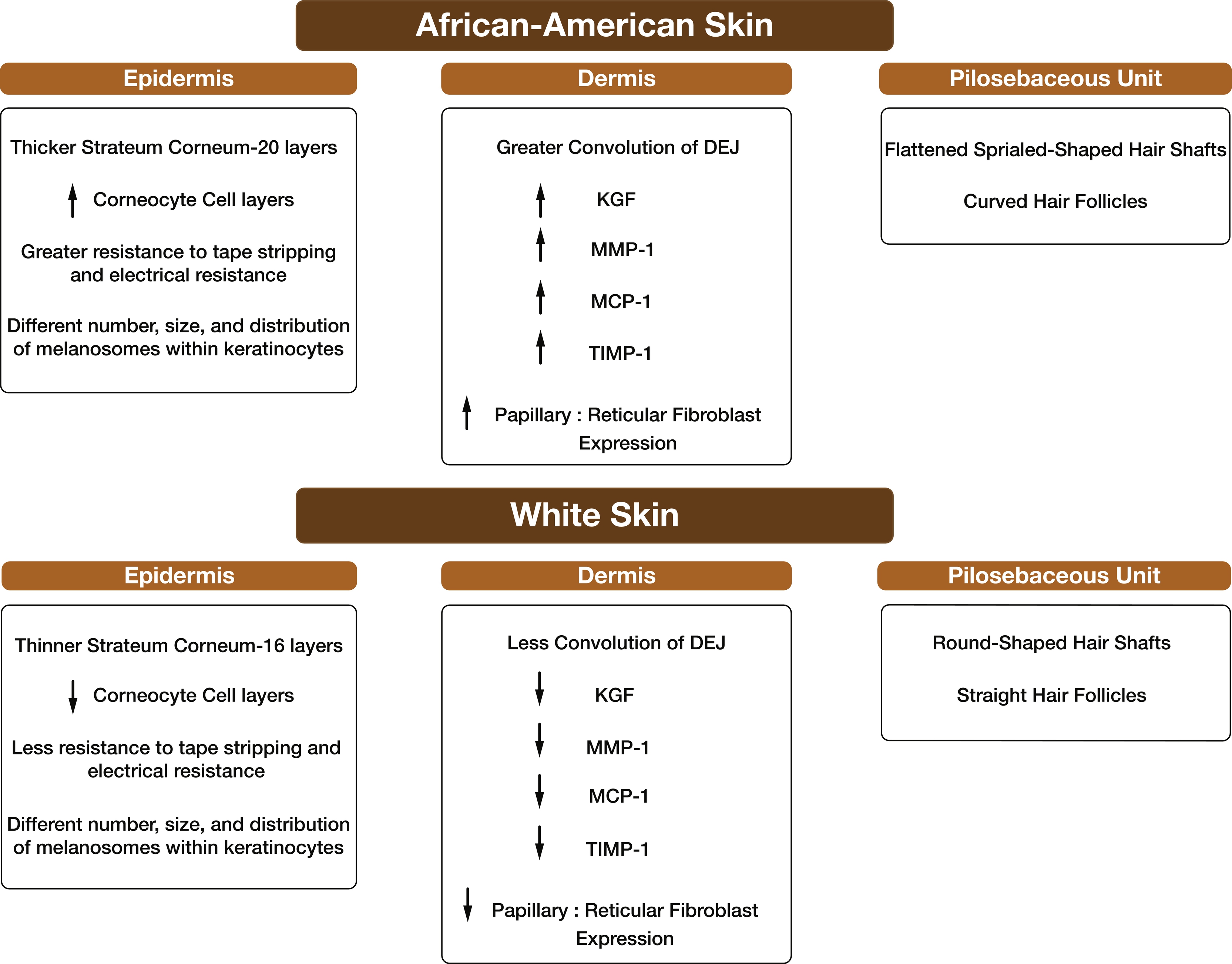
It is useful to consider the histological findings of hidradenitis suppurativa (HS) lesions in the context of populations at risk for HS to understand the clinical differences observed amongst these populations. HS disproportionately affects women, persons 18 to 29 years of age, and African Americans (AA). Women are more likely to have axillary and upper anterior torso involvement, while male HS patients are more likely to have moderate to severe disease and lesions in the perineal and perianal regions. Considering the race discrepancy seen in HS, the histopathology of healthy skin and the pilosebaceous unit is first outlined, highlighting known differences in skin of color ( Fig. 5.1 ). On this background of healthy skin across skin of different colors, the histology of HS is discussed, although differences across the disease spectrum have not yet been studied systematically.
Cutaneous Structure and Cellular Composition in Healthy White and African American Skin
Epidermis
Epidermal thickness (from basal layer to stratum corneum) is location dependent and is considered to be approximately the same in White and African skin. The average epidermal thickness is 100 μm, although anatomical location determines more precise dimensions. AA skin is minimally but significantly 6μm thicker, which may result from differential expression of the dermal papillae and epidermis rete ridges. The main barrier function of the skin lies in the most superficial layer of the epidermis, the stratum corneum. Not only does it protect against chemical injury and microbiologic invasion from the environment, but also maintains water and solute balance. Even though the stratum corneum is equally thick in African American and White skin, African American stratum corneum contains more cell layers (20 layers vs. 16 in Whites). Also, the number of corneocyte cell layers, resistant to tape stripping and electrical resistance, were reported to be greater in African subjects presumably as a result of better intercellular cohesion.
Four major resident populations make up the cells of the epidermis: keratinocytes, melanocytes, Langerhans cells, and Merkel cells. The major populations of epidermal cells are keratinocytes. These cells originate in the stem cell pool in the basal layer and undergo maturation as they migrate upward, ultimately forming the laminated stratum corneum. The human epidermis averages 50 microns in thickness, with a surface density of approximately 50,000 nucleated cells/mm 2 . Under basal conditions, differentiated keratinocytes require approximately 2 weeks to exit the nucleated compartment and an additional 2 weeks to move through the stratum corneum. Markers that identify keratinocytes in normal healthy epidermis are keratin (K) K5 and K14 in basal keratinocytes, and K1, K2e, and K10 in suprabasal keratinocytes.
Melanocytes are pigment producing cells located in the lower epidermis, manufacturing and secreting melanin. The main function of melanin is to protect against UV radiation by absorbing and scattering the rays. Melanocytes package melanin into pigment granules called melanosomes that are transferred by excretion and phagocytosis into nearby keratinocytes. It is the greater number, larger size, increased stability and distribution of melanosomes within keratinocytes that determine differences in skin color, not the number of melanocytes. Some known markers that identify melanocytes are Melan-A, S100 (α and β subunits).
Langerhans cells are the specialized immunologic cells of the skin. They traffic out of the epidermis toward regional lymph nodes, where they play a critical role in antigen presentation during the induction and regulation of skin-directed immune responses. Example of markers that identify human Langerhans cells are CD1a, CD207, and S100 (β subunit).
Merkel cells are tactile cells containing neuroendocrine peptides within intracytoplasmic granules. They are also found in the basal layer of the epidermis. Each Merkel cell is in close contact with a basolaterally settled unmyelinated afferent nerve terminal (nerve plate) that has a high tactile sensitivity to light touching. Markers that identify Merkel cells include cytokeratin (CK) 8, 18, 19, 20, and CD56.
Dermis
The dermis is predominantly composed of connective tissue, collagen, and elastic fibers. The papillary dermis (closer to the epidermis and around adnexal structures) contains mainly collagen type I and, to a lesser extent, collagen type III. The reticular dermis located underneath the papillary dermis contains thick collagen type I. In a histological study comparing the skin of White and African origins, no differences were seen in dermal collagen and elastic fiber organization. However, African skin displayed a greater convolution of the dermo-epidermal junction (DEJ).
Cells found within healthy dermis include fibroblasts (CD90) and immune cells such as macrophages (CD68 and CD163), dendritic cells (CD11c, CD14, CD1c/BDCA-1, and CD303/BDCA-2), and occasional mast cells (CD11b, CD45, CD13, CD29, CD33, CD34, CD41, CD43, CD45, CD117, CD203c, mast cell tryptase). Resident leukocytes are also found in healthy skin, such as T cells and occasional B cells. The pan-T cell marker is CD3, with subsets CD4 helper T cells and C8 cytotoxic T cells. B cells are identified by CD19 and CD20, among others.
Other differences in protein expression have been reported in African skin compared to White skin, including increased keratinocyte growth factor (KGF) and monocyte chemotactic protein (MCP)-1, twofold higher ratio of papillary to reticular fibroblast expression, increased matrix metalloproteinase (MMP)-1, and tissue inhibitor metalloproteinase protein (TIMP)-1 protein expression. In an inflammatory environment, these observations make black skin more prone to pathologies or disorders such as keloids/acne keloids and potentially HS.
Subcutis
The subcutaneous tissue is mainly formed of fibroblasts, lobules of adipose cells separated by collagenous septa that contain the neurovascular bundles, and macrophages.
The Pilosebaceous Unit in Healthy White and African American Skin
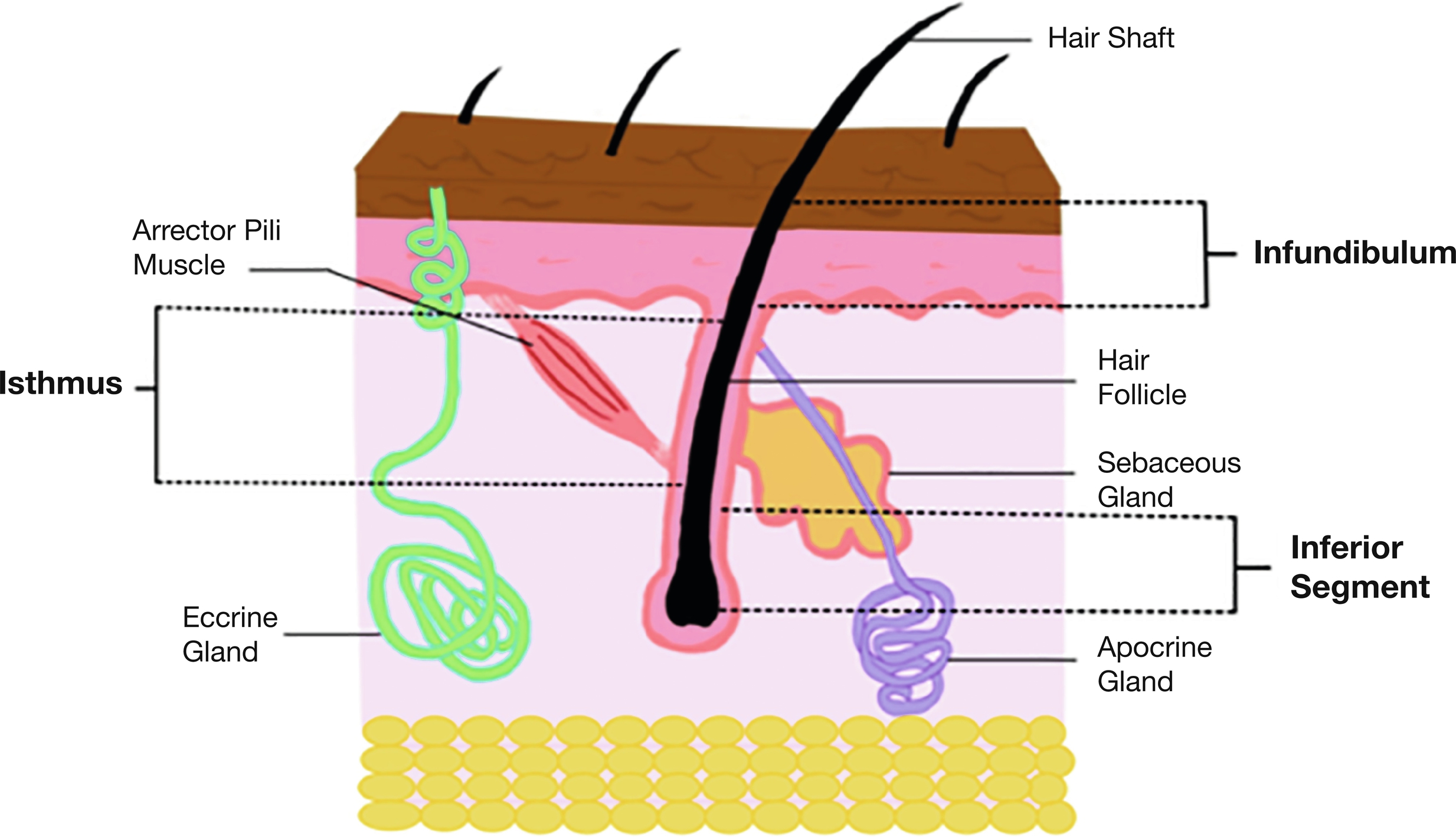
A key component of the clinical diagnosis of HS is the localization of symptoms to apocrine-rich areas with terminal hair follicles, such as the axillae, inguinal and anogenital regions, and perineum. The oil-producing pilosebaceous unit contains a hair follicle, hair shaft, the sebaceous gland, and arrector pili muscles ( Fig. 5.2 ). They are found all over the body except on the hands and feet.
Hair Follicle
Fully developed hair follicles have three distinct areas (see Fig. 5.2 ). The infundibulum extends from the follicular opening on the surface of the epidermis to where the sebaceous duct enters the follicle. Harboring a rich residential microflora and specialized innate immune defenses, the infundibulum acts as a key component of the epidermal environmental interface. Although not well characterized in human skin, K79, LRIG1, and DLX are known transcription factors expressed within the infundibulum of the murine hair follicle. The isthmus extends from the sebaceous duct entry to the insertion of arrector pili muscle. The inferior segment is below insertion of muscle and contains the hair follicle bulb (or matrix), the level where hair cornification starts. The layers of the hair from “inner-to-outer” are the medulla (central part of hair fiber), hair cuticle, inner root sheath (IRS), and outer root sheath (ORS). The function of the tri-layered IRS is to mold the hair by hardening filaments. The ORS forms an outer cylinder support of the hair follicle and is characterized by trichilemmal keratinization. The bulge is a special segment of the ORS near the arrector pili insertion, consisting of a major area of epithelial stem cells of the hair follicle.
The distribution of follicle density at different body sites appears to be the same in all the ethnic skin types. However, there are striking racial differences in hair shaft and follicle shape. African Americans tend to have flattened spiraled hair shafts arising from a curved follicle compared to Whites.
Sebaceous Glands
Sebaceous glands are found in greatest abundance on the face and scalp, although they are distributed throughout all skin sites except the palms and soles. They are composed of lobules of pale-staining cells with abundant lipid droplets in their cytoplasm. They are always associated with hair follicles, draining into the lower infundibulum, except at the following sites: tarsal plate of the eyelids (meibomian glands), buccal mucosa and vermilion border of the lip (Fordyce spots), prepuce and mucosa lateral to the penile frenulum (Tyson glands), labia minora, and female areola (Montgomery tubercles). The sebaceous gland produces and secretes sebum into the follicular duct, a blend of lipids such as triglycerides, squalene, fatty acids, and esters of glycerol. Skin lipids contribute to the barrier function, and some have antimicrobial properties. Antimicrobial lipids include free sphingoid bases derived from epidermal ceramides and fatty acids (e.g., sapienic acid) derived from sebaceous triglycerides.
Apocrine Glands
The straight excretory apocrine duct opens into the infundibular portion of the hair follicle and is composed of a double layer of cuboidal epithelial cells. The coiled secretory apocrine gland is located at the junction of the dermis and subcutaneous fat. It is lined by a single layer of cells, which vary in appearance from columnar to cuboidal. This layer of cells is surrounded by a layer of myoepithelial cells. Apocrine coils appear more widely dilated than eccrine coils. The apices of the columnar cells project into the lumen of the gland and in histologic cross section appear as though they are being extruded (decapitation secretion).
Although occasionally found in an ectopic location, apocrine units of the human body are generally confined to the following sites: axillae, areolae, anogenital region, external auditory canal (ceruminous glands), and eyelids (glands of Moll). Apocrine glands do not begin to function until puberty. Apocrine secretion is mediated by adrenergic innervation and by circulating catecholamines of adrenomedullary origin. Secretions from apocrine glands produce an odor once they are metabolized by skin flora, although the precise function of this is unknown. Interestingly, the scarcity of apocrine glands in the genitofemoral area in which hidradenitis emerges suggests a secondary nature of apocrine involvement.
Eccrine Glands
These glands are found throughout the skin but are most concentrated in palms, soles, and forehead. The eccrine glands, or merocrine glands depicting the gland’s secretion, open directly onto the skin surface by a special spiral intraepidermal duct called the acrosyringium . This is formed by small polygonal cells with a central round nucleus surrounded by abundant pink cytoplasm. The straight dermal portion of the duct is composed of a double layer of cuboidal epithelial cells and is lined by an eosinophilic cuticle on its luminal side. The secretory portion of the gland is formed by an inner layer of epithelial secretory cells surrounded by a layer of flattened myoepithelial cells. The secretory cells are of two types: large, pale, glycogen-rich cells and smaller, darker-staining cells. The pale glycogen-rich cells are thought to initiate the formation of sweat. The darker cells may function similar to cells of the dermal duct, which actively reabsorb sodium, thereby modifying sweat from a basically isotonic to a hypotonic solution by the time it reaches the skin surface. These glands play a major role in thermoregulation and homeostasis.
Sweat is similar in composition to plasma, containing water and the same electrolytes, but in a more dilute concentration. Secretion of hypotonic sweat results in an adaptive response to a thermal stimulus allows greater cooling with conservation of sodium. Anatomically, the amount of sweat glands and sweat pores in African American and White skin is identical and varies with climatic changes but not with racial factors.
Apoeccrine
Apoeccrine glands share some common morphological and functional characteristics with both eccrine and apocrine glands. Their long ducts open directly onto the skin surface like the eccrine sweat duct. The secretory portion of apoeccrine sweat glands consists of a pseudostratified single layer of clear and dark secretory cells surrounded by myoepithelial cells and is irregularly dilated. The dilated segment consists of an apocrine-like single layer of epithelium, but the thin segment of apoeccrine sweat glands shows eccrine sweat gland-like structures. The ratio of apoeccrine to eccrine in axillary skin for adolescent men and women is almost 1:2. However, in some persons, the apoeccrine glands represent less than 10% of the axillary sweat glands. Apoeccrine sweat glands are mainly identified in the axillary and perianal locations and constitute approximately 10% to 45% of axillary sweat glands.
Histological Changes in HS in Skin Structures and Across the Disease Spectrum
Clinico-Pathological Correlation
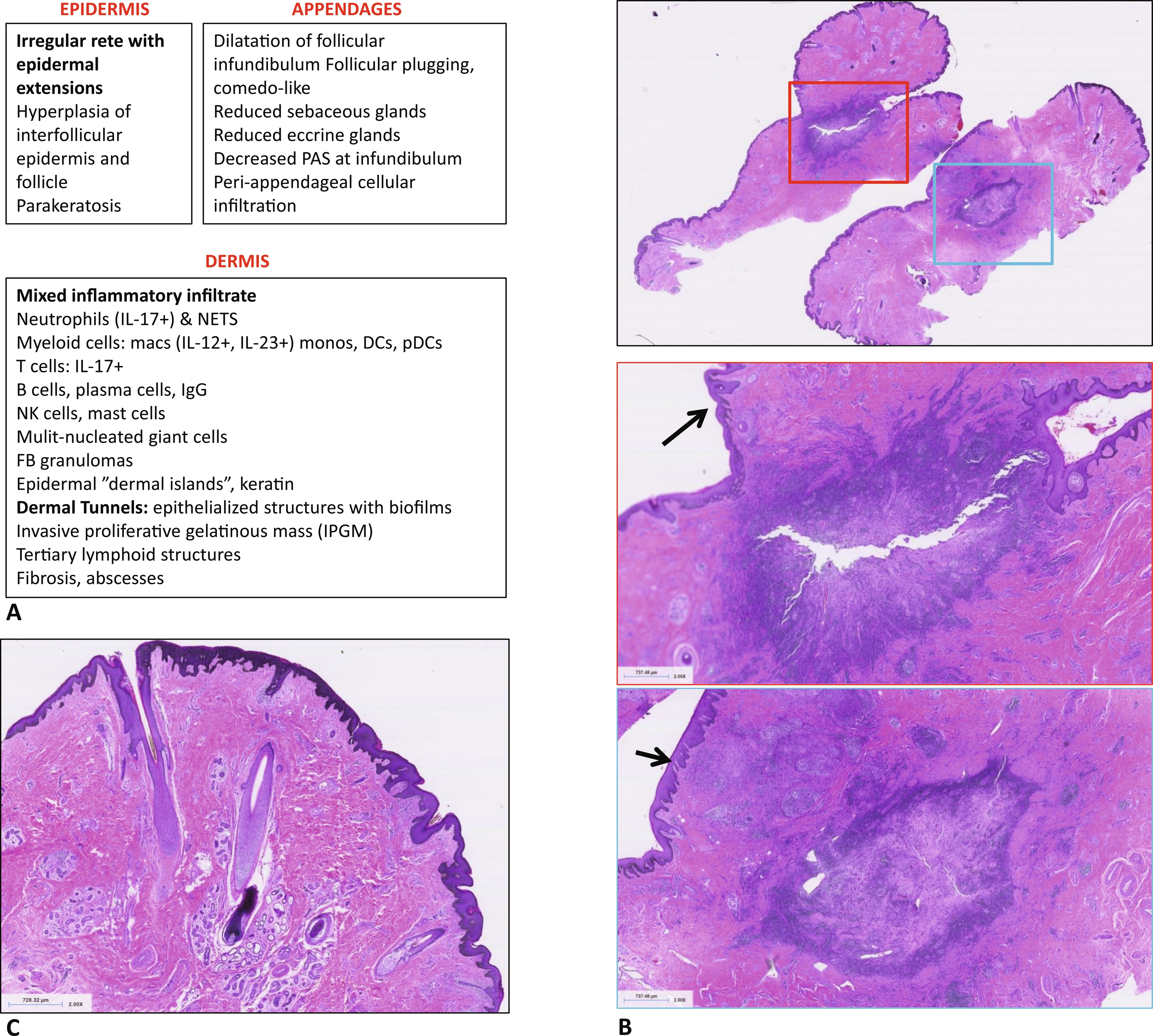
There is no single diagnostic test for HS; rather, diagnosis is based on a combination of clinical and histological features. Below is a general description of histological features seen in skin structures in HS. However, histopathology of specific HS lesions is not well described yet. Nor have there been systematic studies of histological changes as HS progresses or in specific HS phenotypes, such as HS in patients with genetic mutations. The characteristic lesions of HS are inflammatory nodules, abscesses, and dermal tunnels. In early disease there are recurrent nodules and abscesses (Hurley stage 1). As the disease continues, there are persistent nodules and abscesses with dermal tunnels and scarring, separated by normal appearing skin (Hurley stage 2). As HS progresses, whole anatomic regions are affected by nodules, abscesses, extensive dermal tunnels, scarring, and double-headed comedones (Hurley stage 3). Fig. 5.3 lists the known histological features of HS and presents representative histology for established HS lesions.