Introduction
This chapter will focus on the epidemiology of hidradenitis suppurativa (HS), examining the body of evidence available for estimating the incidence and prevalence of HS, and the distribution of HS by gender, age, and race.
Epidemiology is a broad subject that also encompasses the risk factors for disease development and the impact of a disease on societal metrics, such as the likelihood of people with a disease to be unemployed. The intersection of risk factors and disease comorbidities can be difficult to separate, and in this chapter we will discuss correlation and causation studies and how they can provide an idea of which patients are most at risk for the development of HS.
Hidradenitis Suppurativa Epidemiology
Prevalence and Incidence
Prevalence and incidence are the most common metrics used to assess the burden of disease in a population. Prevalence assesses the proportion of a population with a disease over a point in time. In contrast, incidence is the rate of new, or newly diagnosed, cases of a disease over a period of time (typically 1 year). Taken together, incidence and prevalence can show the rate at which a disease is being diagnosed and the duration of time that patients carry the diagnosis. The ability to determine these statistics is affected by data source, coding, and other biases.
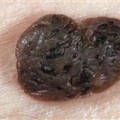
Estimates of the overall prevalence of HS range from 0.03% to 4% with a likely prevalence of around 1% ( Table 2.1 ). Several types of datasets such as those from patient-facing surveys, cohort, and population-based studies have noted differences in prevalence that may be attributed to study design or regional study population differences. Typically, the threshold of 47 cases per 100,000 people or below is defined “rare.” While HS has been classified as a rare disease in the past, increasing data suggest that it should no longer be considered rare.
| Country | Study | Population Queried | Overall Prevalence Estimates | 1-Year Incidence Estimates | Method |
|---|---|---|---|---|---|
| North America | |||||
| US | Lookingbill et al. (1988) | 1,157 | 0.09% | Dermatologist-diagnosed | |
| US | Cosmatos et al. (2013) | 7,927 | 0.053% | Insurance claims database (ICD) codes | |
| US | Kimball and Sung (2013) | 429,329 563,931 | 0.11% in 2007 0.2% in 2011 | Billing codes | |
| US | Shlyankevich et al. (2014) | 2 million | 0.078% | Chart-verified billing codes | |
| US | Shahi et al. (2014) | 144,000 | 0.13% | Billing codes | |
| US | Garg et al. (2017) | 48 million | 0.10% | 0.01% | Chart-verified International Classification of Diseases (ICD) codes |
| South America | |||||
| Brazil | Ianhez et al. (2018) | 17,004 | 0.41% | Survey | |
| Argentina | Zimman et al. (2019) | 143,245 | 0.02% | Chart-verified billing codes | |
| Europe | |||||
| Denmark | Jemec (1988) | 100 | 4% | Survey | |
| Denmark | Jemec et al. (1996) | 507 | 1% 1-year prevalence | Dermatologist-diagnosed | |
| France | Revuz et al. (2008) | 6,887 | 0.97% | Survey | |
| Spain | Albares et al. (2012) | 1,071 | 0.2% | Dermatologist diagnosed | |
| Denmark | Vinding et al. (2014) | 16,404 | 2.10% | Validated survey | |
| Denmark | Riis et al. (2019) | 27,765 | 1.8% | Validated survey | |
| Germany | Kirsten et al. (2020) | 2.3 million | 0.04% | 0.03% | ICD codes |
| Sweden | Killasli et al. (2020) | 9 million | 0.14% | Registry | |
| Africa | |||||
| Mali | Mahé et al. (2002) | 10,575 | 0.03% | Dermatologist-diagnosed | |
| Middle East | |||||
| Israel | Shalom et al. (2015) | 3,207 | 0.7% | (ICD) codes | |
| Asia | |||||
| Korea | Lee et al. (2018) | 50 million | 0.06% | (ICD) codes | |
| Oceania | |||||
| Australia | Calao et al. (2018) | 11,433 | 0.67% | Validated survey | |
Large cohort studies using claims databases or billing codes may underestimate prevalence, as they only capture patients who seek medical care, while survey studies may report higher rates of prevalence, as patients with the disease may be more incentivized to respond. Several methods of analysis exist. Claims database studies use large databases derived from either the provider side or the insurer side. A population-based observational and case-control study using the U.K. Clinical Practice Research Datalink found an overall point prevalence of HS diagnosis to be 0.77%; however, when probable cases (individuals confirmed by primary care physician to have a history of between one and four skin boils in flexural regions) were also included, the prevalence rose to 1.19%. Some studies control for incorrect coding by performing a full or partial chart review to estimate the positive predictive value of each coded diagnosis. For example, a 2017 US population analysis used a chart-verified billing code model to estimate HS prevalence at 0.10% in the US population (98 per 100,000 persons).
Several smaller cohort studies have used dermatologist-rendered diagnosis instead of billing code diagnosis resulting in prevalence estimates ranging from 0.03 to 0.2% (see Table 2.1 ). Survey studies report a higher estimate of prevalence on average, with the notable caveat that survey validation may influence the resultant reporting. Studies performed in Denmark and Australia used previously validated HS surveys to report a general prevalence of 0.67–2.1%. Other survey studies, using unvalidated questionnaires, have reported prevalence ranging from 0.41% to 4%.
Breakdown of prevalence by disease severity is rarely reported, as it is difficult to ascertain through billing codes and patient surveys. However, one US analysis of predominantly white patients in Olmsted County, Minnesota, reported a distribution of Hurley stage as Hurley I 59.7%, Hurley II 38.1%, and Hurley III 2.2%. Smaller studies of HS patient cohorts have observed a higher burden of moderate to severe HS with over half of patients in Hurley stages II and III, and high-severity patients may be overrepresented in samples drawn from patients seeking care.
As awareness of the diagnosis rises, there may also be an increase in incidence. Only two studies have reported HS incidence (see Table 2.1 ). Through a US dataset, the 1-year incidence of HS in the United States was reported as 11.4 per 100,000 (0.01%), with the 10-year incidence as 8.6 per 100,000. In Germany, the incidence was estimated at 0.03%.
Demographic Factors
When evaluating the epidemiology of a disease, it is important to consider the factors that influence disease prevalence, as they may give insight into the types of populations more susceptible to the disease and help determine populations that should receive increased screening or for whom clinicians should have a higher baseline suspicion of disease. Prevalence of HS has been evaluated against different demographic factors and found to vary based on gender, age group, and race ( Table 2.2 ). Several studies examining this demography in more detail have provided insight as to the types of populations likely to contain the highest disease burden.
| Demographic Factor | Hidradenitis Suppurativa Associations |
|---|---|
| Gender |
|
| Age |
|
| Race |
|
Gender
Most Western studies note an increased prevalence in female versus male HS patients, but, gender predominance may differ based on racial or geographic differences, as researchers studying Asian populations report male predominance.
For example, in the United States, female patients had a prevalence of more than double that of males: 137 per 100,000 compared with male patients at 58 per 100,000. This is echoed in other studies, including a Swedish study, that showed a prevalence of 0.21% in women versus 0.07% in men. Brazilian population surveys also showed a higher prevalence among females, 0.49%, versus a prevalence of 0.30% among males. The proportion of female/male was found to increase with age group from 0.46% female/0.38% male for age below 40 years to 0.56%/0.23% for age above 40 years. In the HS UNITE registry, which draws on populations from North America, Europe, and Australia, 69.7% of registrants were female.
Notably, while most population-based analyses are age-adjusted, in an American analysis limited to the pediatric HS population, there remains an increased prevalence of female versus male patients at 0.045 to 0.012, respectively. This proportion has also been observed in a smaller-scale single-center study of the US pediatric HS population, which reported 85.7% versus 14.3% female to male patients.
One Korean retrospective study of 438 participants noted a higher ratio of male patients, 2.5:1, and noted that male patients had more severe disease. Similarly, a larger Korean population-based analysis found that males predominated with a ratio of 1.6:1. A questionnaire-based study in Japan also noted a higher disease burden in male patients with a ratio of 2:1 and a small study of 58 patients in Singapore also reported a higher percentage of males (58.6%). Further population-based studies are needed to clarify this difference, but it may be that the gender differences in HS prevalence are specific to certain populations or genetic variance.
Race
It is increasingly well-documented that HS disproportionately affects non-Asian minorities. Several studies in the United States support this conclusion. Garg et al. found that the prevalence of HS in African Americans was threefold higher than white patients, 296 per 100,000 compared with 95 per 100,000. While few true prevalence studies exist that examine race, the racial makeup of HS patients has been proxied by comparing the race of patients seeking care for HS versus other conditions. In one US single-center analysis of 476 HS patient charts, 66% were African American, despite making up only 36% of patients seen at the study institution. In another US single-center analysis of 366 HS patients, 6.4% of black patients seeking care were for HS complaints, while only 3.9% of white patients seeking care were for HS. A retrospective review in the United States of 284 HS patients found an even more stark ratio of 7.22:1 African American to white HS patients. Data from the National Ambulatory Medical Care Survey were also examined to show the odds ratio (OR) for African Americans compared with white patients seeking care for HS as 2.00. A 2014 review of data from the National Center for Health Statistics found that 23% of visits for HS are from African American patients, compared with 13% of visits from African American patients overall. In addition, in one retrospective study of 375 patients, African American patients were significantly more likely to present with more advanced Hurley stage II or III disease compared with all other racial groups (OR 2.46, P = .003).
Overall, studies in the United States support the higher burden of HS disease in African American patients. It remains to be seen whether this is an example of variations in HS by race that is replicated in other countries. While reported prevalence of HS varies somewhat by region, further population-based analyses are needed before we can concretely determine more affected populations.
Age
Disease prevalence also varies by age, with the highest prevalence in the United States seen between age 30 and 39. This is generally the age range cited as when disease onset most commonly occurs, though one French study reported the typical median age of onset for HS symptoms as between 20 and 24 years.
The age of onset distribution of HS is usually reported as unimodal within the age range of 30 to 39 years, but one study reported a bimodal age distribution observed with a second peak of self-reported disease symptom onset in post-menopausal women. This finding corresponds with an observation from a Brazilian study that the ratio of women to men increases with age, implying that if there is a late-onset group of HS disease, it is predominantly experienced by women.
Several studies have reported that up to 25% of HS cases may present before age 18, which some authors have qualified as “early onset” or “adolescent” disease. One Spanish study of 134 HS patients reported 51.5% experiencing adolescent-onset disease, and in Brazil, a higher prevalence of HS was found in adolescents (0.57%) as compared with adults (0.47%). One study also reported that racial differences in age of onset may be observed, suggesting that, similar to the differences seen in gender-based analyses based on country where the study was conducted, age-based prevalence analyses may also be affected by the demographics of the population studied. In a US pediatric analysis, prevalence was highest in female adolescents aged 15 to 17 years. Recently it has also been suggested that early-onset HS patients are more likely to be overweight or obese.
There is a documented delay in diagnosis of between 7.2 and 10.2 years for HS patients. An association of diagnostic delay of HS with increased disease severity has been suggested. These delays in care may also impact our assessment of HS severity prevalence, as patients have cited that the first 6 years of disease are typically the most severe, and these are the years that are delayed to care.
Considering the overall prevalence of HS in childhood in contrast to adult-diagnosed HS, so-called “early-onset HS” continues to meet the threshold of classification for a rare disease, with fewer than 47 cases per 100,000 people.
Risk Factors
Risk factors are another cornerstone of epidemiology research, as some risk factors may be modifiable. In addition, risk factors also continue to help determine populations who may benefit from increased screenings and other disease-modifying interventions. Several risk factors, both behavioral and genetic, have been associated with HS. In most cases, causation has not yet been established, as the pathogenesis of HS remains under investigation. Most risk factors correlate only to the presence of HS; however, some are associated with an increase in disease severity.
Family History
No dominant genetic pattern has yet been established for HS, although several small genome-wise association studies (GWAS) have now been published. Only one gene, a gamma-secretase, comprising four units—presenilin (PSEN), presenilin enhancer-2 (PSENEN), nicastrin protein (NCSTN), and anterior pharynx defective-2—has been associated with 5% of HS families who exhibit a dominant inheritance pattern. The majority of studies examining this gamma-secretase unit have associated HS disease with mutations in either PSEN, PSENEN, or NCSTN, but these mutations do not account for disease in the vast majority of HS patients. In addition, one other study notes that a higher copy number of defensin genes, which encode for proinflammatory defensins, may be related to HS inheritance. Currently, the consensus regarding HS inheritance is that it is a genetically heterogenous disease.
Up to 35% of HS patients have been reported to have a positive family history of the disease, and a positive family history of HS has been associated with an earlier age of disease onset compared with the commonly cited range of 20 to 24 years. There may be overlap between the prevalence of “adolescent onset” disease and a positive family history of HS. Interestingly, there have also been reports that an absence of family history correlates with increased disease severity.
Family history of HS may also vary based on ethnicity, similarly to the observed differences in disease prevalence. A case series of 58 Japanese patients found that only 1.7% of Japanese patients had a family history of HS, which was significantly lower than the 29.1% observed in data the authors amalgamated from Western studies.
Lifestyle
Many different facets of lifestyle, including smoking, obesity, and socioeconomic status, have been linked with HS ( Table 2.3 ). While study design varies, most studies report an “increased risk” of HS in groups with the associated risk factor. This may be reported as an increased likelihood, or in an OR, a measure of association that compares the odds of disease in those with the risk factor to the odds of disease in those without the risk factor.