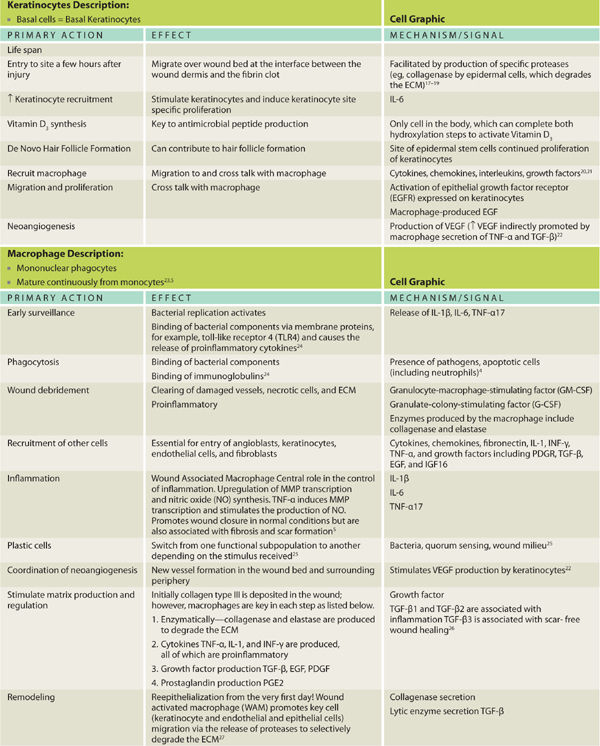
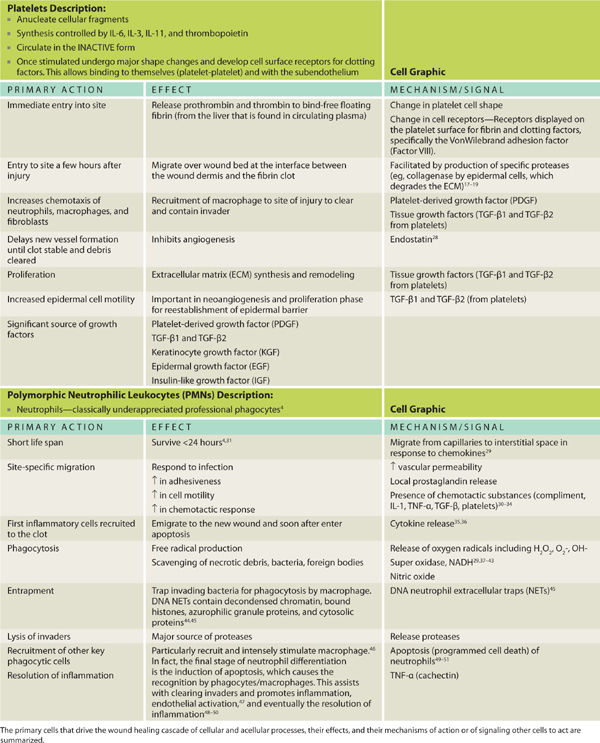
Phenotypical changes in prominent cells are important in directing healing throughout the influence and production of many of the chemical messengers as illustrated. Highlighted cells presented include the platelet, macrophage, and fibroblast. The macrophages have a pronounced cellular functional metamorphosis and are the central orchestrators of the healing process. Cellular roles pertinent to wound healing are depicted in TABLE 2-1 along with the fluid interconnected communication and cell signaling that occurs to effect progression through the healing process.
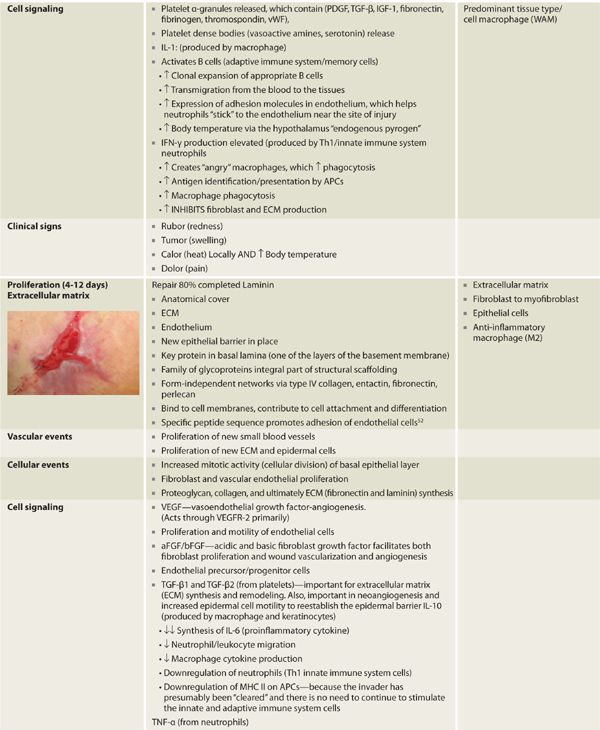
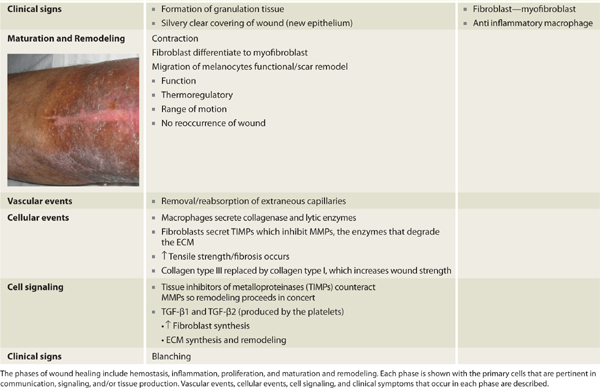
The pivotal cells and phases of healing are depicted in TABLE 2-2, which provides a cross-reference of healing phases with key cells and signals, as well as a chronological timeline, thus providing the reader an appreciation of the overlapping and essential functions directed in concert as opposed to an isolated or oversimplified view of cell function. Although exceedingly complex, the elegance lies both in the ability of multiple systems to evoke healing and the use of paracrine, autocrine, and juxtacrine mediators to effect expedient resolution of tissue injury using the resources immediately available.
TABLE 2-2 Phases of Wound Healing
Cells exhibit various levels of activity in response to many factors. FIGURE 2-1 summarizes four recognized levels of activity and the associated effect on the local environment. Cells that exist in a senescent state (defined as resistance to apoptosis or programmed cell death) disrupt normal tissue differentiation, drain the metabolism, and secrete cell products that negatively impact the wound environment. Cells in a baseline state have normal mitotic and metabolic activity, actively survey and monitor adjacent tissues, and do not impact surrounding tissues negatively. An up-regulated cell has a higher level of metabolic activity and purposefully responds in concert with other cells in reaction to injury, presence of pathogens, or both. A cell that is “out of control” exhibits an overproduction of cellular byproducts, is not coordinated with any other cells, and does not respond to feedback inhibition. The cartoons that represent each level of cell activity are overlaid in important diagrams to help the reader discern the cellular state in normal and disrupted wound healing. Both the correct cells and the appropriate level of cellular activity are required to ensure wound healing.
FIGURE 2-1 Cell activity levels
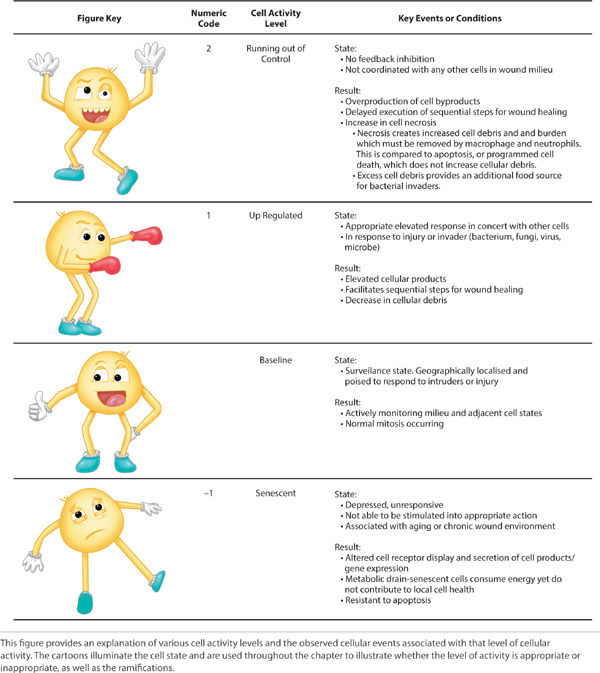
FIGURE 2-2 provides an illustration of the intricate and exquisite concert of cell migration, proliferation, and signaling in the context of cell signaling and vascular events, all working in unison to culminate in healing.
FIGURE 2-2 Cell migration, proliferation, and signaling in the wound healing process
HEALING RESPONSE
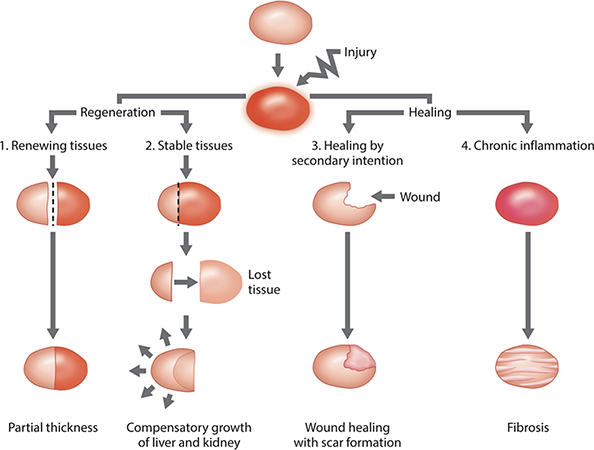
The healing response occurs by one of the following four mechanisms: (1) continuous cell cycling, (2) cell proliferation, (3) regeneration, or (4) fibroproliferative response. Normal intact skin is representative of continuous cell cycling whereby labile cells are constantly undergoing a balance of proliferation and programmed apoptosis throughout life, thereby resulting in a steady-state. The basal keratinocytes continuously undergo mitosis (cell division), followed by migration to the skin surface, and finally sloughing. Cell proliferation occurs when the damaged or lost tissue is replaced by the expansion of remaining healthy cells that undergo mitosis. The structure is not completely duplicated; however, function is approximated.
Regeneration occurs with the loss of a structure. The acute injury that undergoes regeneration stimulates complete duplication in both structure and function of the lost tissue. The liver, hematopoietic tissue, gastrointestinal tract epithelium, and epidermis are examples of tissue that are capable of regeneration. Fibroproliferative healing typically occurs in dermal wound healing. The lost tissue is not replaced, but rather a “patch” is constructed that restores the skin covering, integrity, and function. Inflammation that fails to progress to healing results in fibrosis. This chapter discusses the phases of wound healing and the involved cells, as well as the mechanisms by which acute and chronic wound healing occur. Divisions of wound healing are graphically depicted in FIGURE 2-3.
FIGURE 2-3 Divisions of wound healing
Classifications of Healing Response
Wound healing can be classified by category or by depth; both assist clinicians in communicating clearly regarding patient needs. Categorically, four divisions describe wound healing: categories 1 to 3 describe healing of full thickness wounds while category 4 refers to partial skin thickness wounds.53,54
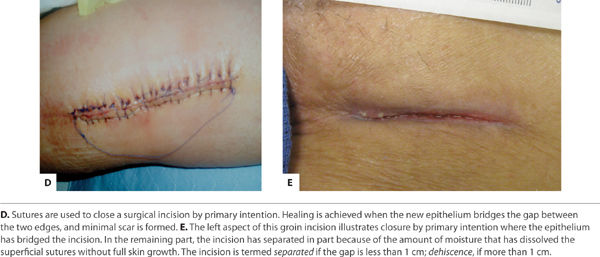
Category 1 Category 1 (Primary Intention) occurs when a clean surgical incision is created and the resulting wound is free from contamination of bacteria, fungi, or foreign bodies. There is minimal tissue loss and the edges can be safely approximated and secured with sutures, staples, or surgical glue. The clotting cascade at the wound surface is largely not initiated and the resulting fibrous scab is absent because of the minimal mortality of cells central to wound healing. The cell signaling cascades usually launched in an acute penetrating injury are not activated. This incisional wound resolves in an orderly, sequenced manner over the course of approximately two weeks.55 (FIGURE 2-4)
FIGURE 2-4 Healing by primary intention
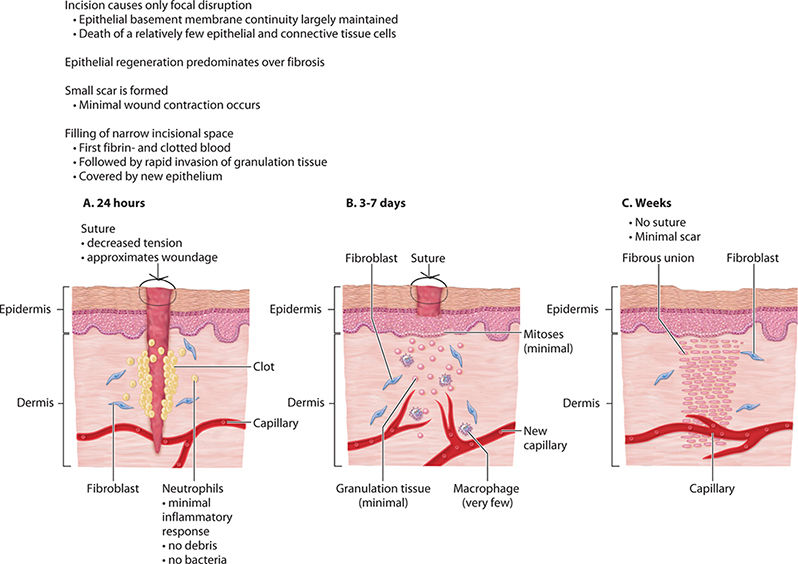
Category 2 Category 2 (Delayed Primary Intention) occurs when wound edges are not approximated because of the concern for the presence of pathogens or debris, an abscess, or in the case of extensive tissue loss (FIGURE 2-5). Delayed primary wound healing is set in motion by the release of multiple pro-inflammatory cytokines, chemokines, and growth factors. Foreign debris is walled off by macrophages that may metamorphose into epithelioid cells, which in turn become encircled by layers of mononuclear leukocytes. The layers of mononuclear leukocytes can be compared to the sequential layering of nacre on a pearl—the clam overlays the grain of sand with nacre, smooth and protecting. In a wound, the result is a granuloma, with the foreign body or pathogen at the center, walled off from the host tissue. In these wounds the inflammatory response is more intense and is accompanied by increased granular tissue formation.55
FIGURE 2–5 Healing by delayed primary intention Large wounds can be partially closed with retaining sutures, or in the case of this abdominal wound, with tension sutures. This technique is used if approximating the edges puts too much strain on the periwound skin and subcutaneous tissue or if there is concern for infection and drainage that needs to be removed in order to prevent abscess formation.
Frequently these wounds may undergo a delayed surgical closure after surgical removal of the granuloma, abscess, or debris. Primary closure may also be completed surgically as a follow-up to negative pressure wound therapy. Once the wound is determined to be ready for closure, surgical intervention, such as suturing, skin graft placement, or flap design is performed, provided wound edges can be approximated. If the host-initiated cleansing, termed autolysis, of the wound is incomplete, chronic inflammation can ensue. The result, without further intervention, is likely to be prominent scarring.
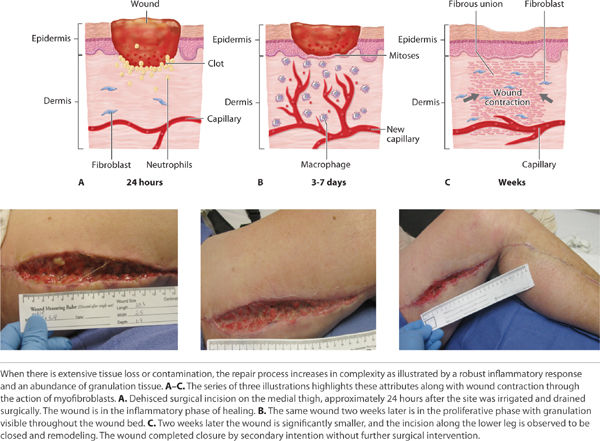
Category 3 In Category 3 (Secondary Intention) healing is entirely accomplished through an appropriate inflammatory response, granulation tissue formation and re-epithelization. Left to close without surgical intervention, wound contraction by myofibroblasts plays a significant role. The myofibroblasts have characteristics of smooth muscle cells and, when activated, they contract and thereby assist in consolidating the extracellular matrix and decreasing the distance between the dermal edges. The myofibroblasts are maximally present in the wound from approximately 10 to 21 days post-wounding.14 Additional time may be required for these wounds to close depending on the surface area and depth (volume) of the wound.55 (FIGURE 2-6)
FIGURE 2-6 Healing by secondary intention
Category 4 Partial thickness wounding refers to the loss or partial loss of the epidermis and or the epidermis and superficial dermis (the basement membrane is intact and the hypodermis is not exposed). In this case healing is accomplished by the process of epithelial cell mitosis and migration. Wound contracture is not an expected or common occurrence during the healing process of these wounds, as the sub-dermal layers are not involved and minimal to no granulation tissue is formed (FIGURE 2-7).55
FIGURE 2–7 Healing of a partial-thickness wound Re-epithelialization can be seen at the wound edges, as well as on the small “epithelial island” at the lower edge. The island indicates that the epithelial cells in that region are migrating from the hair follicle rather than the edge and is commonly seen in partial-thickness wounds.
Wounding can also be categorized by the depth and involvement of tissue lost or damaged as a result of the injury. These classifications progress from the most superficial erosion, to partial thickness of the dermis, and full thickness extending into the hypodermis. Chapter 1 explains this in detail with correlation to the appropriate skin structure.
Overview of Healing
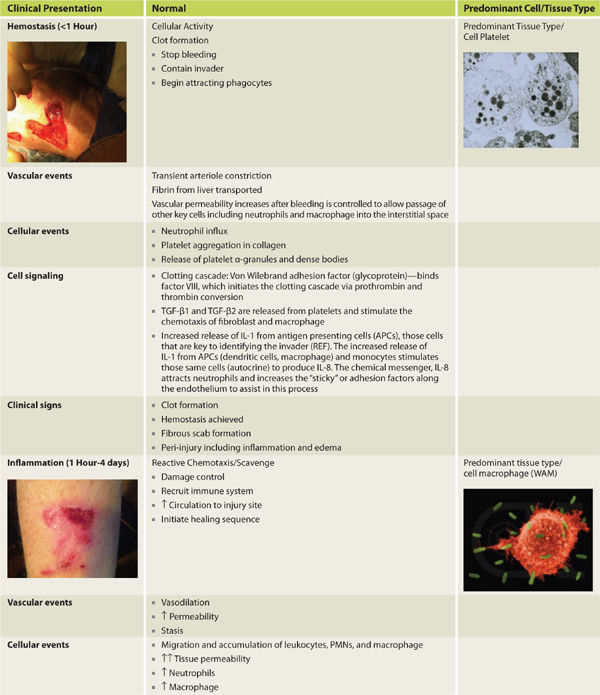
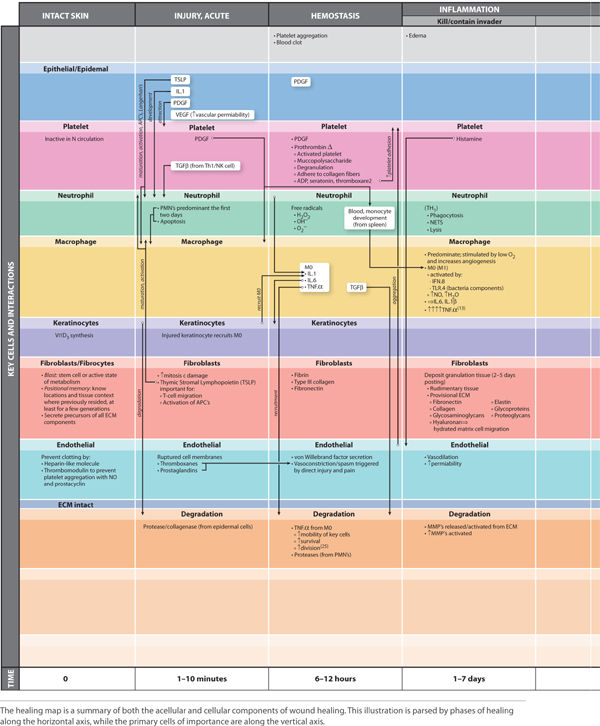
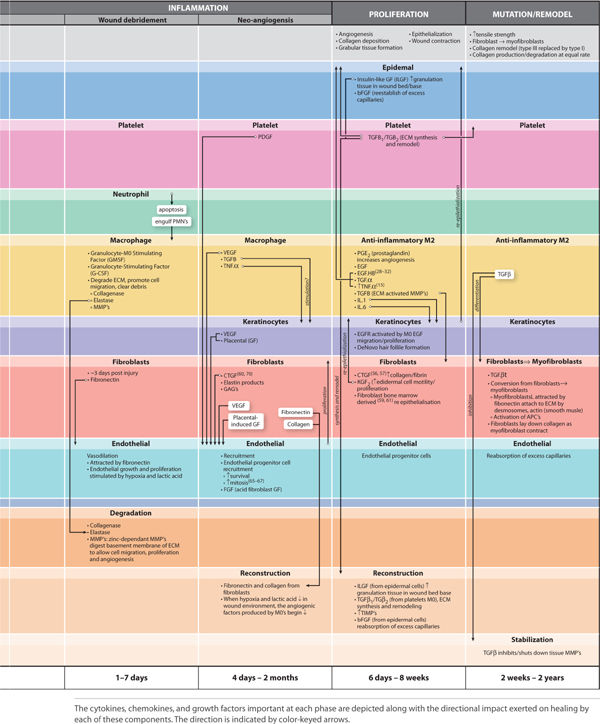
Acute wound healing is subdivided to the following phases: hemostasis, inflammation, proliferation, and remodeling. Inflammation is further subdivided into three overlapping phases: kill/contain the invader, inflammation, and neo-angiogenesis. FIGURES 2-8 to 2-14 provide a framework for each of the major healing phases in terms of four important events: (1) vascular, (2) cellular, (3) cell signaling, and (4) clinical signs. FIGURES 2-8, 2-9 depict normal intact skin prior to wounding, FIGURE 2-10 is hemostasis, FIGURES 2-11 to 2-13 depict inflammation, and FIGURES 2-14 to 2-16 represent proliferation. The interplay between each of the four events is both complex and elegant. Each of these four events is explained in detail, along with their contribution to normal healing.
FIGURE 2–8 Normal intact skin
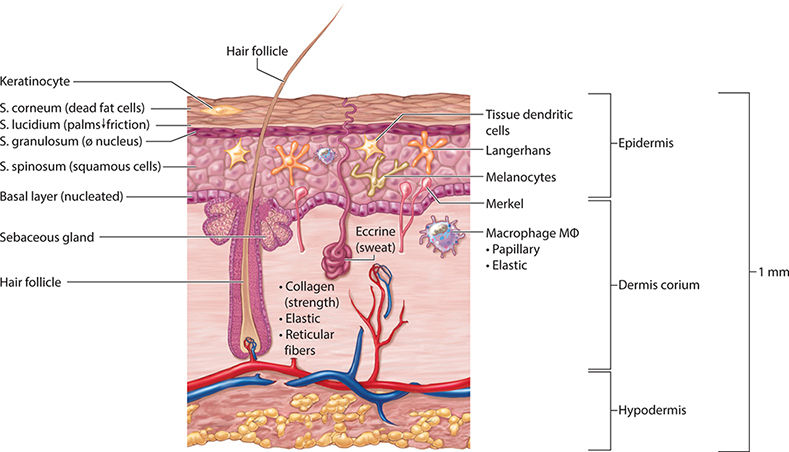
FIGURE 2-9 Baseline state of the skin prior to injury
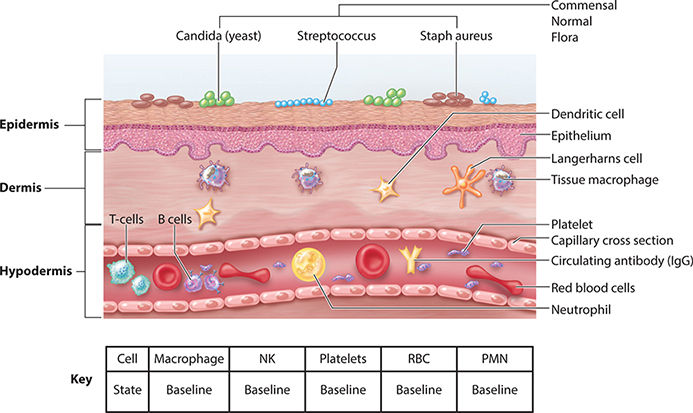
FIGURE 2-10 Hemostasis
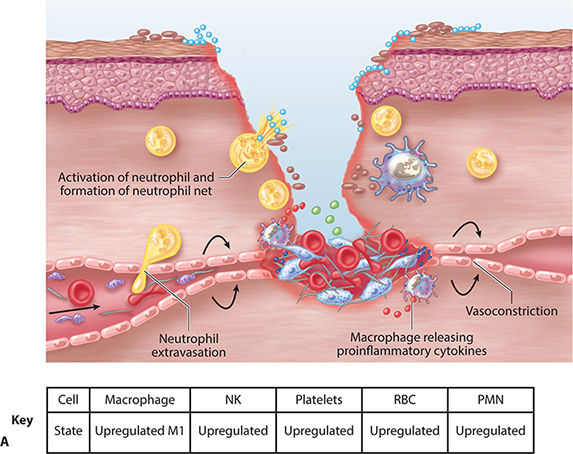
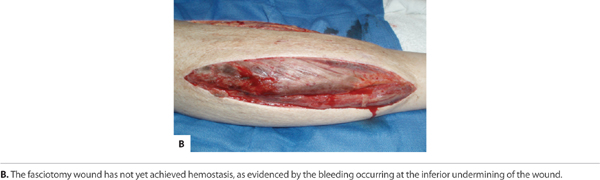
FIGURE 2-11A Inflammatory phase: contain and kill the invader
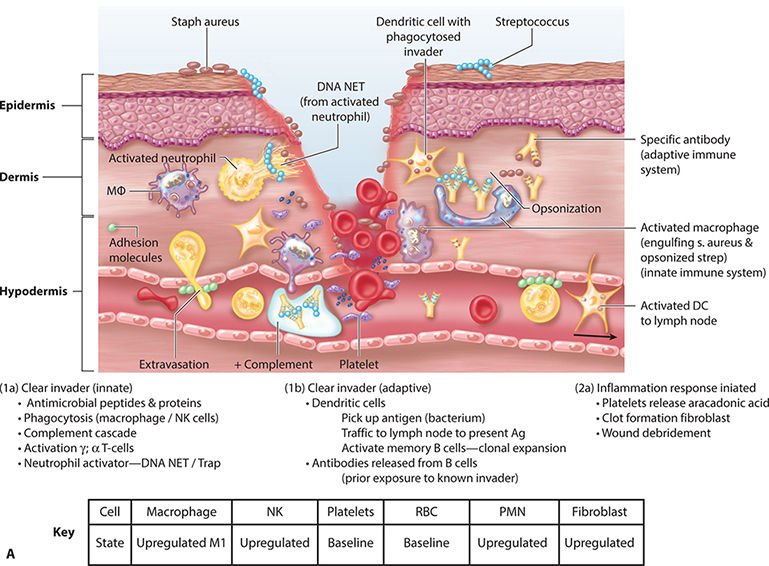
FIGURE 2-11B Inflammation: killing the pathogens
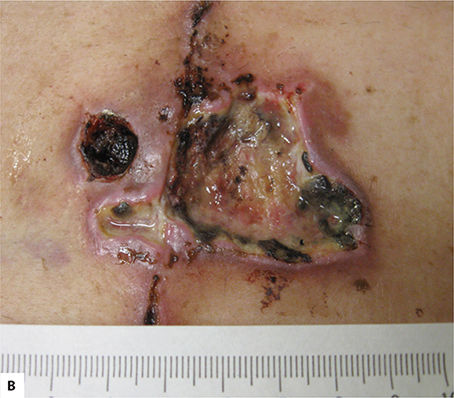
FIGURE 2-12 Inflammatory phase: wound debridement
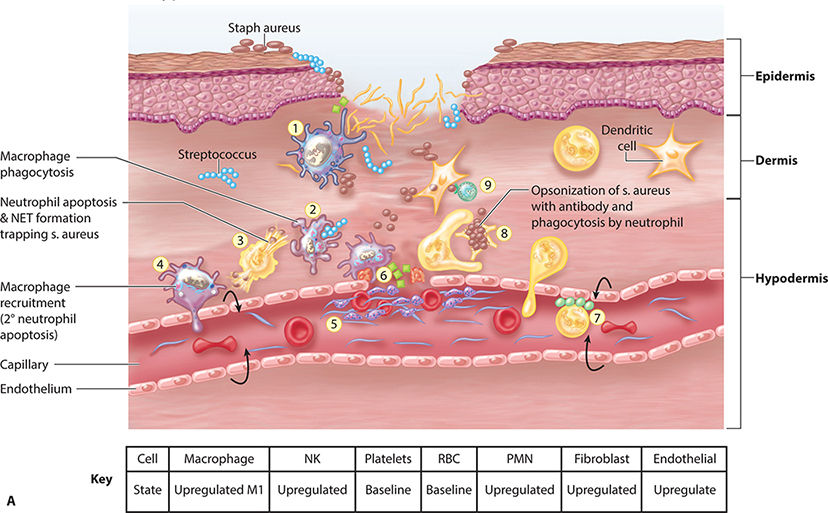
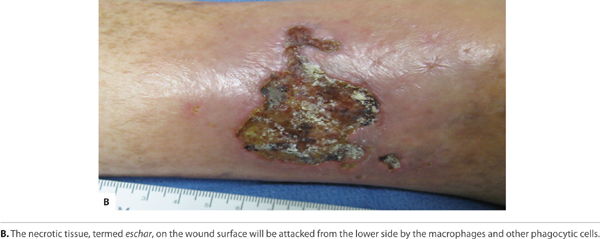
FIGURE 2-13A Inflammatory phase: neo-angiogenesis
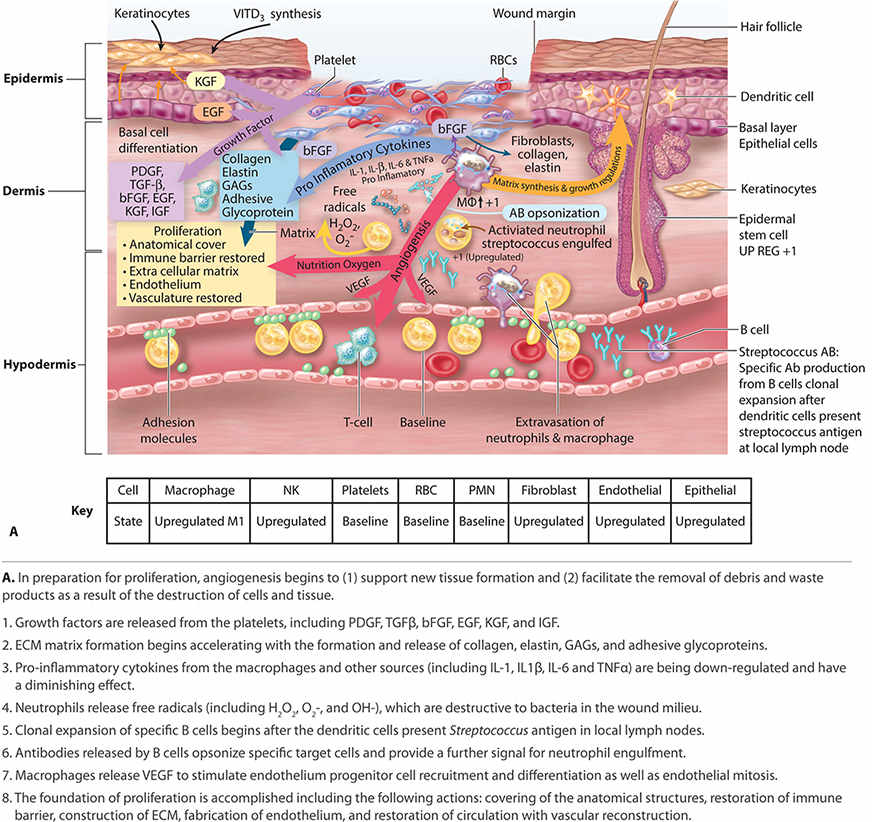
FIGURE 2-13B Neo-genesis
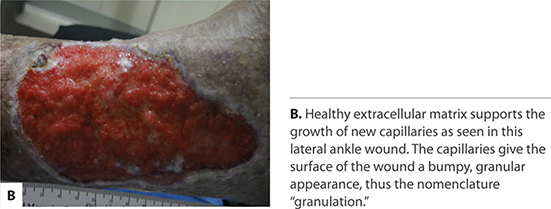
FIGURE 2-14A Proliferation
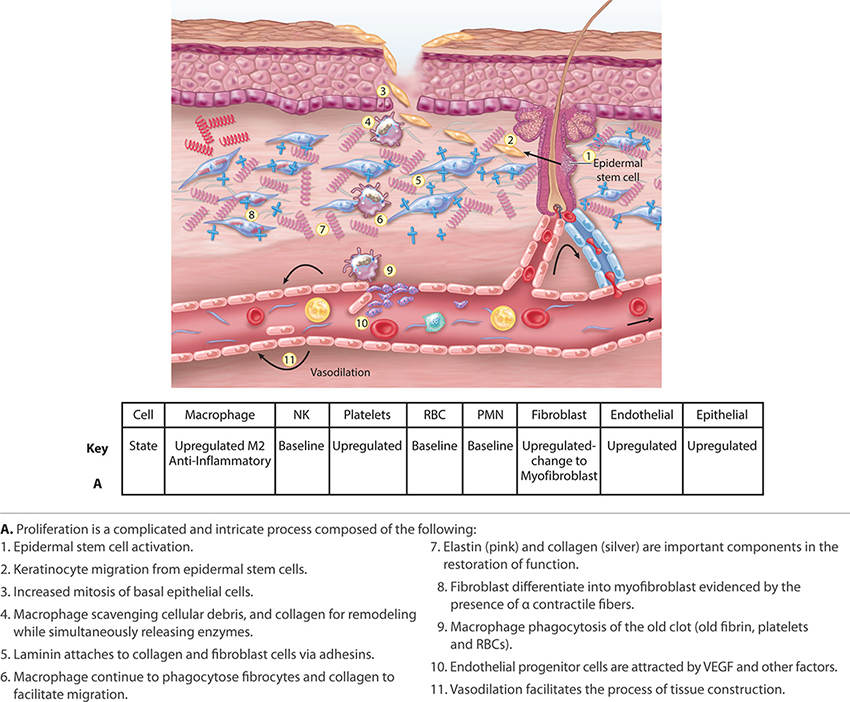
FIGURE 2-14B Proliferation
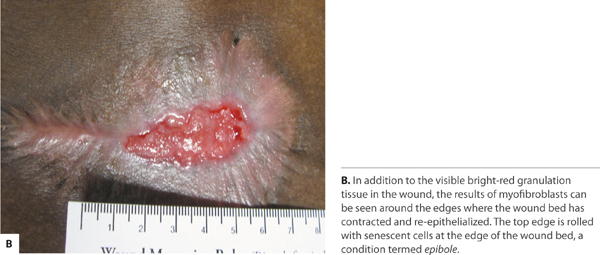
FIGURE 2-15 Angiogenesis
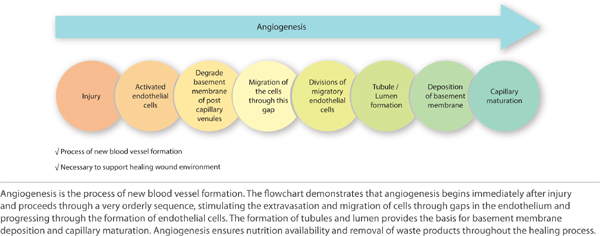
FIGURE 2-16 Proliferation
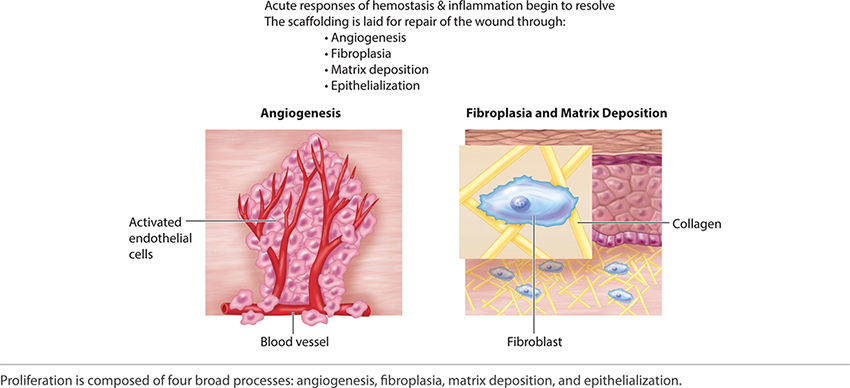
1. Vascular events include hemostasis, transient vasoconstriction, retrograde degradation of damaged vessels, and the transition to endothelial cell differentiation, migration, and proliferation—also termed neo-angiogenesis.
2. Cellular events include the directed migration and accumulation of cells known to be necessary for wound healing (eg, neutrophils and macrophages) to the site of injury.5 A fascinating characteristic of some cells includes their ability to morph in phenotype and function, depending on the phase of healing and the surrounding stimuli, whether cytokine, chemokine, or ECM activation.5
3. Cell signaling orchestrates healing by these mechanisms: cytokines, chemokines, growth factors, and receptor accessibility on target cells. It is accomplished through the binding of chemical messengers to cell receptors displayed on the target cell surface. The binding of the chemical messenger (eg, cytokine, chemokine, growth factor, or interleukin) activates or depresses target cell DNA transcription and protein translation of important activities that are performed by the target cell. These activities include (a) the production of additional chemical messengers, (b) a change in cell phenotype and function, or (c) binding to adjacent ECM sites. Cell signaling occurs between cells (cell to cell) and between the cell and wound matrix (cell to matrix).56
4. Clinical signs and symptoms are those observable changes in the local periwound environment (pain, redness, and edema) or patient systemic symptoms (fever, chills, increased heart rate, or pain).
Cytokines are small proteins or glycoproteins that are secreted by numerous cells and alter the function of the target cell. The target cell for the cytokine/interleukin may be itself (autocrine) or a neighboring cell (juxtacrine). Cytokines can be either pro-inflammatory or anti-inflammatory.
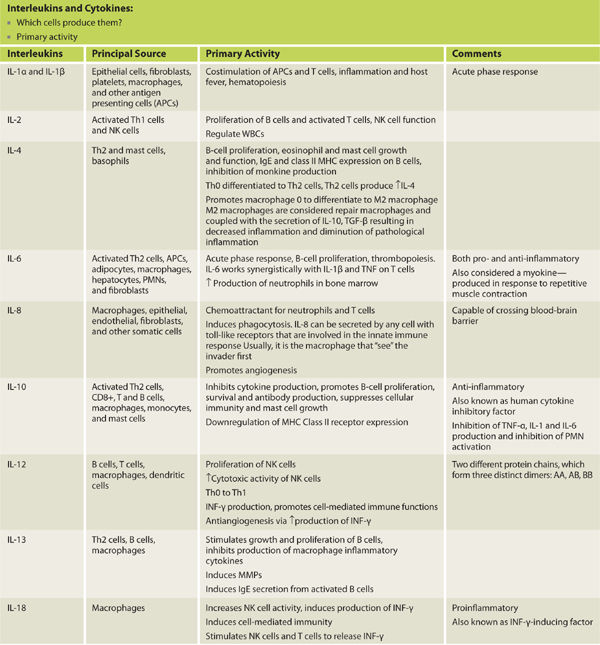
Interleukins are a group of cytokines that were first observed being expressed by white blood cells (leukocytes).1 The term interleukin derives from inter- as a means of communication, and -leukin, deriving from the fact that leukocytes produce many of these proteins and are the target of their action. The name is something of a relic as it has been determined that interleukins are in fact produced by a wide variety of body cells. The function of the immune system depends in a large part on interleukins. The majority of interleukins are synthesized by helper CD4+ T-lymphocytes, as well as monocytes, macrophages, and endothelial cells.3
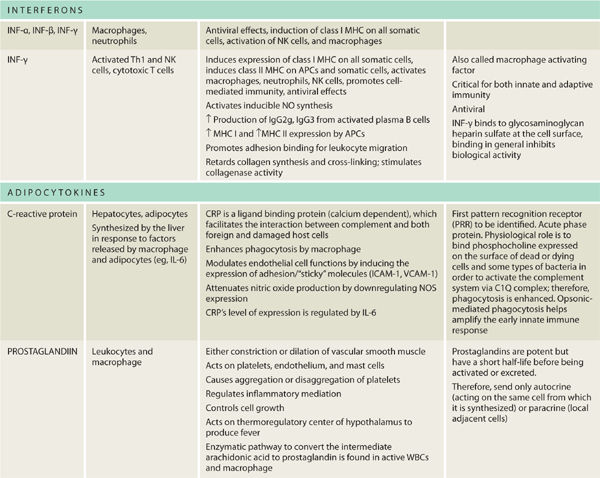
TABLE 2-3 lists the cytokines important to wound healing. The pro-inflammatory cytokines necessary for wound healing are: TNF-α, IL-1, IL-2, IL-6, IL-8, and IFN-γ. In general, IL-4 and IL-10 are considered anti-inflammatory. Receptor expression on target cells can be either up- or down-regulated. Each of the signals and the complex interplay serves to enhance, depress, or change entirely the cell function while ensuring that the process culminates in functional wound healing.
TABLE 2-3 Principal Sources and Primary Activities of Interleukins and Cytokines
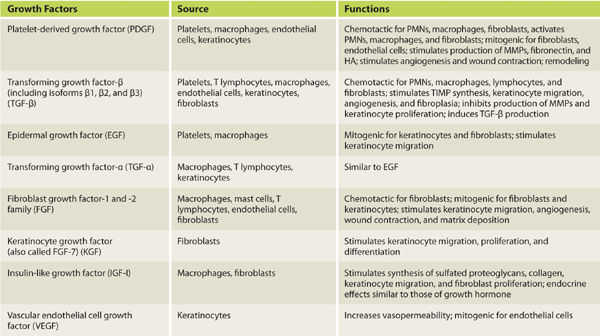
Growth factors are soluble polypeptides, produced in both normal and wounded tissues, which stimulate cell migration, proliferation, and alterations in cellular function. They are extremely potent and can exert significant effects in nanomolar concentrations. Growth factors bind to specific cell receptors and have one of two different effects: (1) the stimulation of DNA transcription or (2) the regulation of cell entry in the cell cycle (mitosis). Growth factors important in wound healing are listed in TABLE 2-4.
TABLE 2-4 Cell Source and Function of Growth Factors Involved in Wound Healing
Cell-to-wound matrix communication occurs extensively during debridement and angiogenesis. Matricellular proteins destabilize the cell–matrix bonds and interactions, in essence creating a more fluid environment for cell migration. Proteinases, both plasminogen activators and matrix metalloproteinases (MMPs), act to dissolve the wound matrix both immediately after injury and during the proliferative and remodeling phases. This is necessary during angiogenesis for the directed migration of endothelial cells.
In order to understand the intricacies of the communication, the components of the extracellular matrix (ECM) in their entirety must be understood. Components of the ECM include fibrous structural proteins, water-hydrated gels, and adhesive glycoproteins. The fibrous structural proteins include the collagens and elastins, which confer tensile strength and recoil to the tissue. Water-hydrated gels that permit resilience and lubrication are categorized as proteoglycans and hyaluronans. Adhesive glycoproteins connect the matrix elements to one another and to cells. The common components of the ECM are illustrated in FIGURE 2-17.
FIGURE 2-17 Extracellular matrix
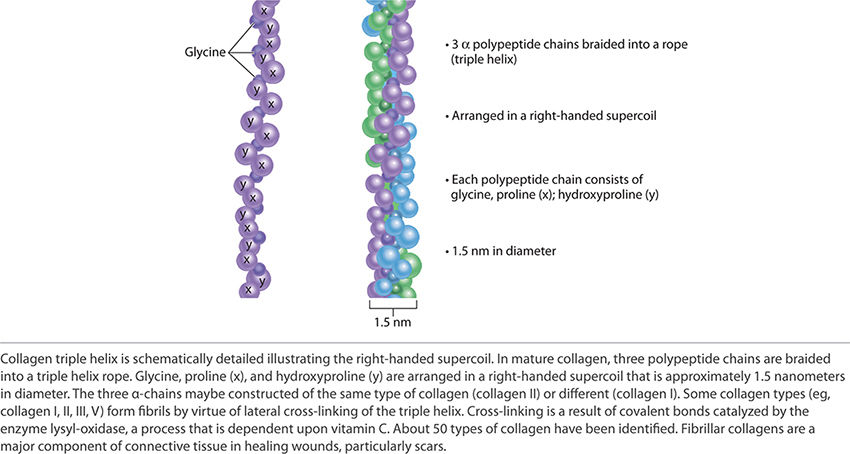
Collagen, one of the two fibrous structural proteins, is composed of three separate polypeptide chains braided into a rope like a triple helix. FIGURE 2-18 depicts the triple helix structure of collagen. There are approximately 50 types of identified collagen. Some collagen types (eg, I, II, III, V) form fibrils by virtue of lateral cross-linking of the triple helix. These collagens form a major portion of connective tissue in healing wounds and particularly in scars. The cross-linking is a result of a covalent bond catalyzed by the enzyme lysly-oxidase. This process is dependent on vitamin C. Types of collagen important to wound healing include: Type I—skin and bone, Type IV—basement membrane, and Type VIII—dermal–epidermal junction.
FIGURE 2-18 Collagen triple helix
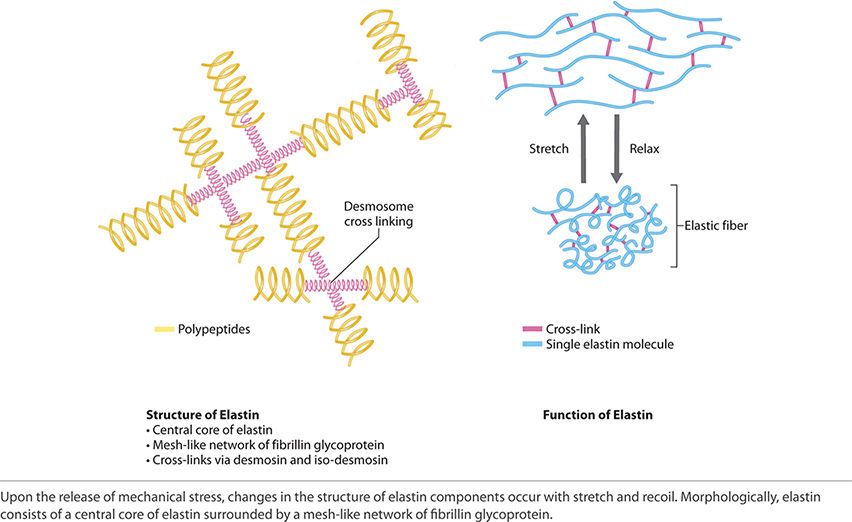
Elastin is present mainly in the skin, large vessels, ligaments, and uterus. Morphologically, elastin consists of a central core of elastin surrounded by a mesh-like network of fibrillin glycoprotein. Fibroblasts secrete fibrillin into the ECM where it becomes assimilated into insoluble microfibrils and provides a platform for elastin deposition. FIGURE 2-19 shows the structure and ability of elastin to stretch and recoil.
FIGURE 2-19 Elastin structure and function
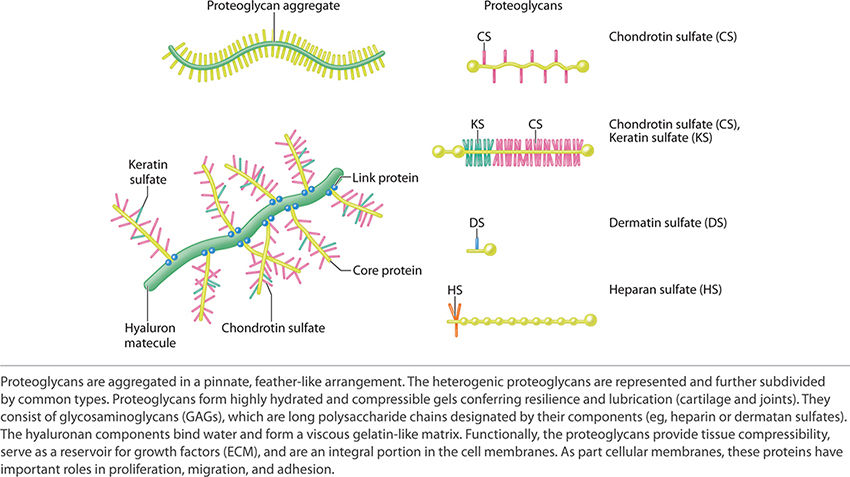
Proteoglycans form extremely hydrated compressible gels that provide both resilience and lubrication (eg, in the skin, cartilage, and joints). Proteoglycans consist of glycosaminoglycans (GAGs) and hyaluronan. GAGs are long polysaccharide chains like heparin sulfate and dermatan sulfate. Hyaluronan binds water and forms a very viscous, gelatin-like matrix. Proteoglycans function to provide compressibility and serve as a reservoir for growth factors that are secreted into the ECM. Proteoglycans are also an important component of cell membranes and as such have roles in proliferation, migration, and adhesion. See FIGURE 2-20 for examples and for the schematic structure of proteoglycans.
FIGURE 2-20 Proteoglycan structure
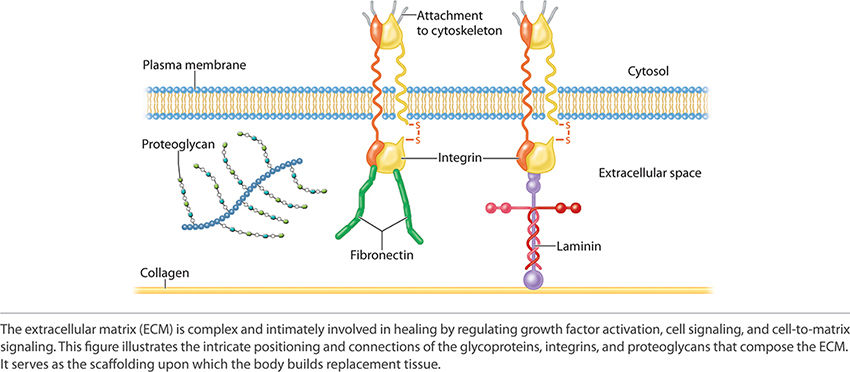
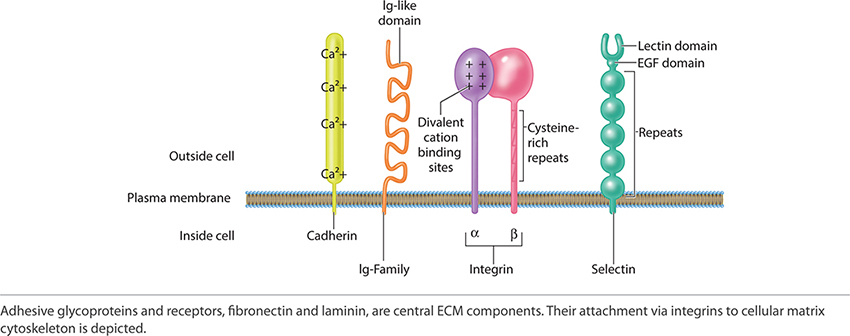
The adherent components of the ECM are the adhesive glycoproteins and adhesion receptors. The adhesive glycoproteins include fibronectin and laminin. The adhesion receptors include immunoglobulin, selectin, cadherins, and integrins. Together laminin and fibronectin, by adjoining to collagen and connecting to the cellular plasma membrane of cells primary to tissue healing, reestablish both strength and function to the new replacement tissue. Laminin and fibronectin form the critical active junction providing both orientation and a dynamic functioning framework. See FIGURE 2-21 depicting the adhesion receptors, which are paramount to ECM function and structure.
FIGURE 2-21 Adhesion receptors
PHASES OF ACUTE WOUND HEALING
Wound healing initially appears so very simple.57–59 The human body is designed to heal, repair, and in some cases regenerate lost tissue through a well-orchestrated sequence of events. When it proceeds as planned—though infinitely complex—healing is at once elegant, rapid, and efficient. The following phases of wound healing are delineated by the pertinent vascular, cell-signaling, cellular activity, and clinical response as previously defined.
Hemostasis—Clot Formation
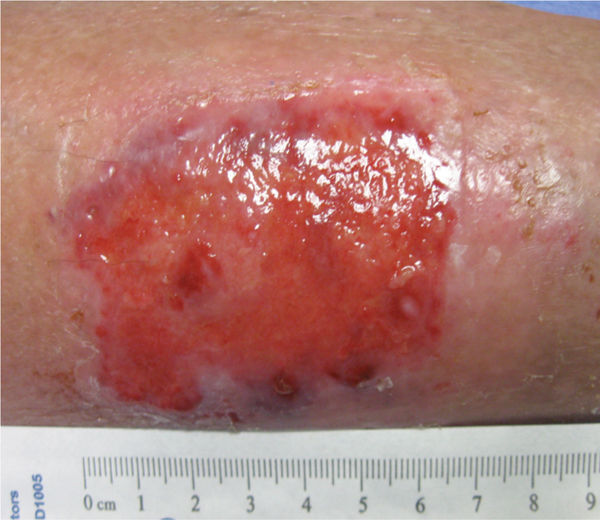
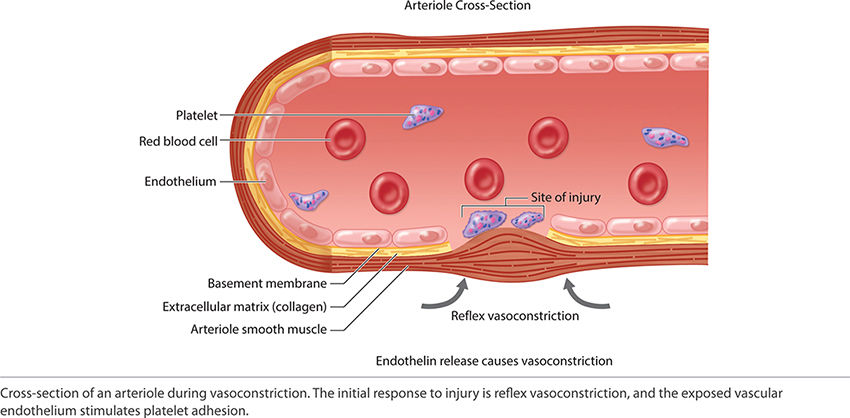
With an acute injury, the small blood vessels respond initially with vasoconstriction to stem further blood loss and tissue injury. (FIGURE 2-22) Activated platelets adhere to the endothelium and eject adenosine diphosphate (ADP), which promotes the clumping of thrombocytes and further ensures clot formation. The clot is composed of various cell types, including red blood cells, white blood cells, and platelets. The clot is stabilized by fibers of fibrin.17 (See FIGURES 2-23 and 2-24).
FIGURE 2-22 Vasoconstriction
FIGURE 2-23 Primary hemostasis
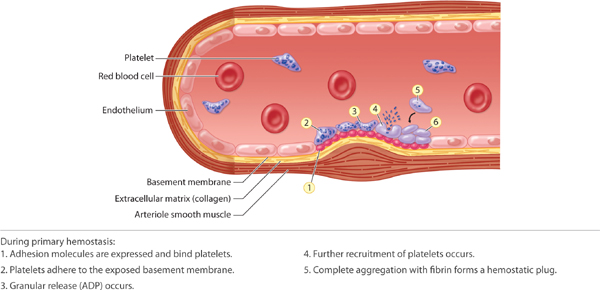
FIGURE 2-24 Stable fibrin clot, or coagulum The dark red fibrin clots on the surface of this skin tear prevent further bleeding and tissue damage.
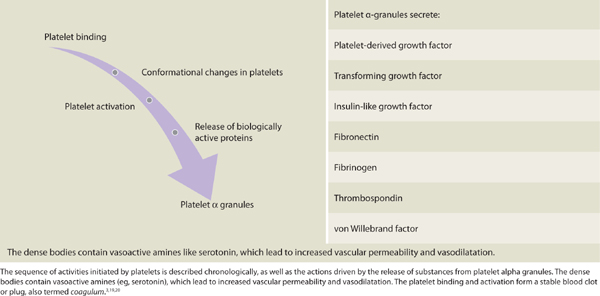
Alpha granules containing platelet-derived growth factor (PDGF), platelet factor IV, and transforming growth factor beta (TGF-β) are liberated from the platelets. Dense bodies contained within the thrombocytes release vasoactive amines, including histamine and serotonin. PDGF is chemotactic for fibroblasts, and in coordination with TGF-β modulates mitosis of fibroblasts,60 thereby increasing the number of fibroblasts in close proximity to the wound. Fibrinogen is cleaved into fibrin. Fibrin undergirds the structural support for the completion of the coagulation process and provides an active lattice for the important cellular components during the inflammatory phase. The fibrin will further serve as a scaffold for other infiltration cells and proteins. (See TABLE 2-5.3,10,61) Clinically, a full thickness wound bed with a stable clot functions to mitigate blood loss. Clear proteinaceous exudate may or may not be present.
TABLE 2-5 Platelet Binding and Activation
Inflammation
Inflammation presents clinically as rubor (redness), tumor (swelling), calor (heat), and dolor (pain). These signs along with the cellular mechanisms responsible are highlighted in the tables, figures, and text that follow. At the immune cellular level several cells arrive, depart, up-regulate, or down-regulate during the phases of healing. The orchestrated arrival and departure of important cell mediators is depicted in FIGURE 2-25. Important changes that permit the initiation of inflammation and result in the clinical findings associated with inflammation are summarized in TABLE 2-6.
FIGURE 2-25 Arrival and departure of migratory cell during wound healing
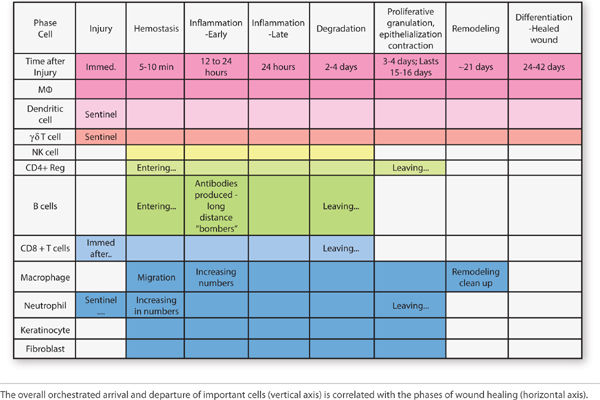
TABLE 2-6 Inflammation Overview
Kill and Contain Invader Within the first six to eight hours after injury, polymorphonuclear leukocytes (PMNs) or neutrophils flood the wound. TGF-β (released from platelets) facilitates PMN migration and extrusion from surrounding intact blood vessels to the interstitial wound space. PMNs are phagocytic cells, functioning to cleanse the wound of debris, including both necrotic cells and pathogens. The highest number of PMNs within the wound is observed between 24 and 48 hours post injury. By 72 hours, PMN numbers are significantly reduced, just as macrophages are infiltrating.56,62,63 FIGURE 2-26 summarizes factors that promote neutrophil adherence and migration. These factors are a combination of cellular activity, chemokines, cytokines, and proteases. TABLE 2-7 summarizes PMN function and cellular events in healing.
FIGURE 2-26 Factors related to neutrophil migration and adherence
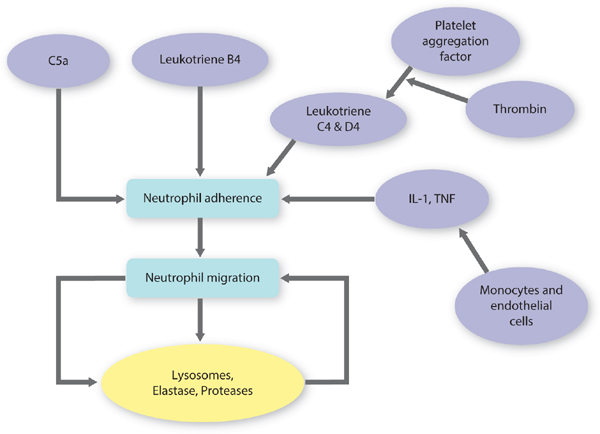
TABLE 2-7 Polymorphonuclear Cells in Wound Healing
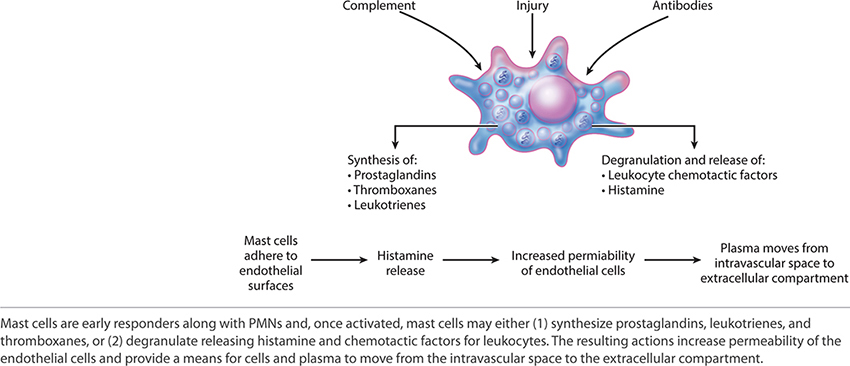
Mast cells are activated by antibodies and move rapidly from vascular circulation to the site of injury. Upon activation, mast cells become “sticky” and adhere to the endothelial surface. (FIGURE 2-27) Mast cells can influence the local environment by degranulation or through the products that they synthesize. Histamine, a product of degranulation, increases the permeability of endothelial cells, thus facilitating extravasation of PMNs and macrophages. The three major product categories synthesized by mast cells are prostaglandins, thromboxanes, and leukotrienes.3
FIGURE 2-27 Mast cell structure and function
Neutrophils play a noteworthy role in the early phagocytosis of pathogens and in the recruitment of macrophages by programmed apoptosis. Neutrophils are also capable of expelling a neutrophil extracellular trap (NET) that is composed of DNA and loosely aggregated chromatin. The NET is somewhat like flypaper, trapping would-be invaders and allowing increased clearance by recruited macrophages or neighboring neutrophils.45
Wound Debridement Autolytic wound debridement begins immediately post-injury and includes the use of cells and enzymatic proteins to break down necrotic tissue. Cells typically involved in debridement include neutrophils, macrophages, and mast cells.36,64 Enzymatically active proteins, including proteinases and collagenases, degrade the damaged tissue and ECM, thus providing a migratory path to the site of injury for the key cells needed to complete the healing process.17,19
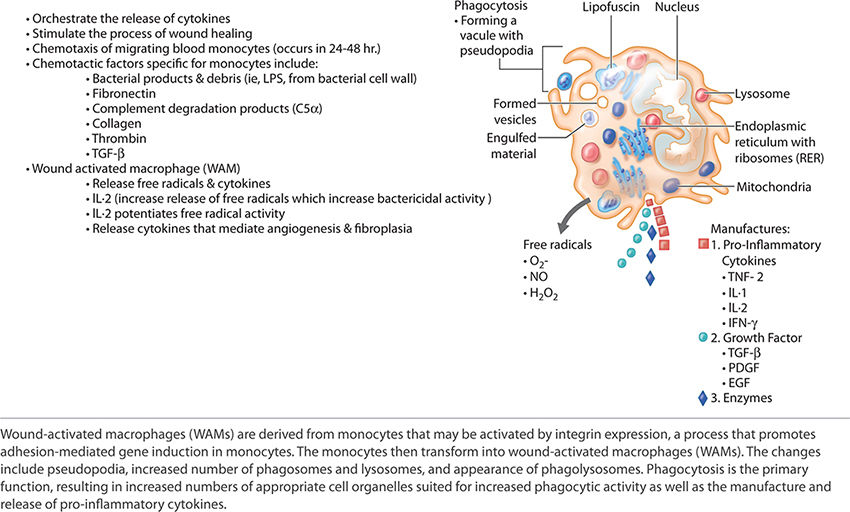
PDGF, released by platelets, is chemotactic for monocytes, causing the monocytes to exude from adjacent vessels and transform into wound-activated macrophages (WAMs). WAMs continue the process of debris removal, and more importantly, begin the signaling that will orchestrate the remaining transitions and events central to healing. (FIGURE 2-28)27,60 Macrophage numbers will remain high for approximately three to four days, releasing various tissue growth factors, cytokines, interleukin-1 (IL-1), tumor necrosis factor (TNF), and PDGF.63 In addition to the phagocytic function, the macrophages provide a unifying script for the multiplication of endothelial cells and the sprouting of new blood vessels, both of paramount importance for continued cell migration and proliferation.3,5,65–67
FIGURE 2-28 Wound-activated macrophage functions
The controlled liquefaction of the ECM continues during the debridement process until all of the damaged cells are removed and cells central to repair are in place. The rate of autolytic debridement will decline as debris is cleared, new cells proliferate, and healing ensues. The pro-inflammatory cytokines and their effects are found in TABLE 2-3 by cell and in TABLE 2-2 by phase of wound healing.
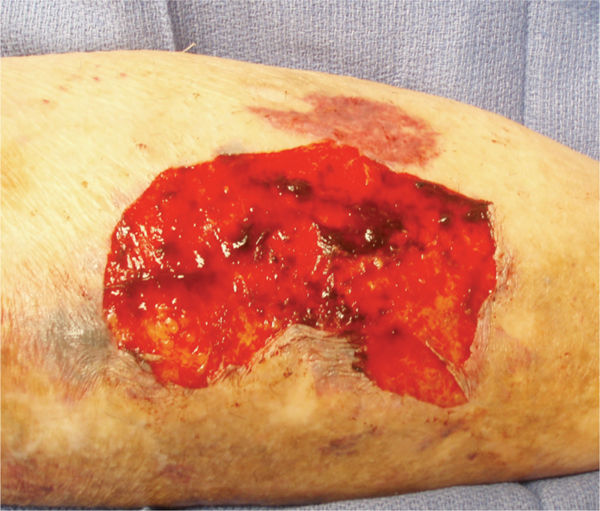
Angiogenesis (Early) Just as the re-epithelialization process begins a few hours after wounding, so, too, does neo-angiogenesis.68 Keratinocytes from the wound edges migrate beneath the fibrin clot and over the wound. Activated fibroblasts migrate to the site of injury in response to TGF-β1,11,60,69 and, in combination with macrophages, form granulation tissue.16,68 Both macrophages and fibroblasts produce vascular endothelial growth factor (VEGF). In addition, fibroblasts produce connective tissue growth factor (CTGF),70 which results in their proliferation via an autocrine loop.12 The formation of new vasculature requires both extracellular matrix and basement membrane degradation followed by migration, mitosis, and maturation of endothelial cells. Basic FGF and VEGF are believed to be central in modulating angiogenesis.10,28,71–74 As a critical component of healing, new vessel formation will both (1) supply the oxygen and nutrients required and (2) remove the waste products of autolysis. FIGURES 2-29 and 2-30 provides a broad overview of the key events involved in angiogenesis while clinically a well-vascularized, early granulating wound bed.
FIGURE 2-29 Angiogenesis overview
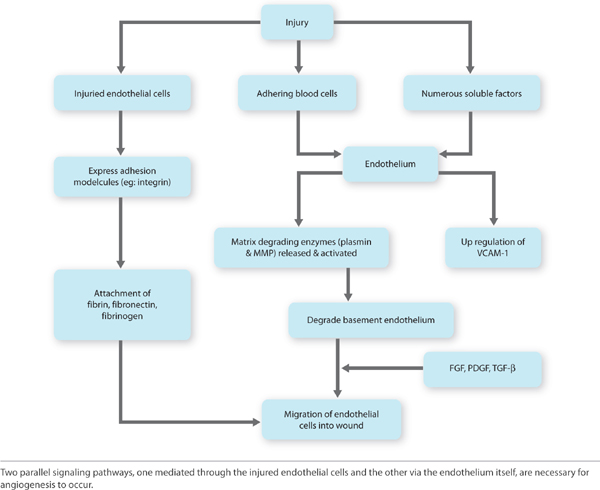
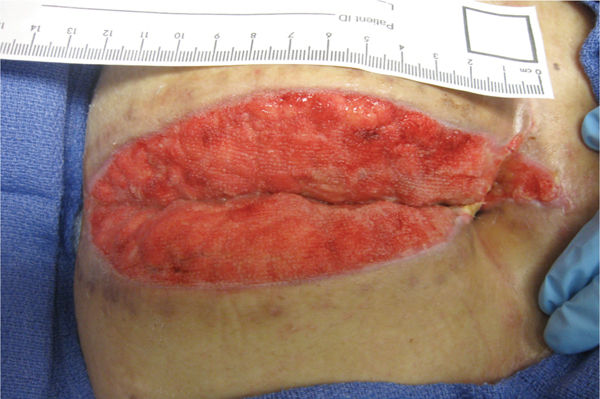
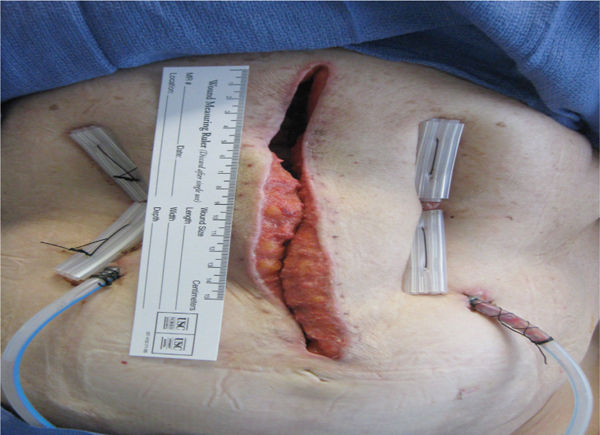
FIGURE 2-30 Granulating wound bed When the signaling pathways occur in a timely sequence, healthy, beefy red granulation forms in the wound bed, supporting the migration of epithelial cells at the edges.
Proliferation
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


 Increased permeability is due to
Increased permeability is due to The combination of intense vasodilation and increased vascular permeability leads to clinical findings of inflammation
The combination of intense vasodilation and increased vascular permeability leads to clinical findings of inflammation PMNs are not essential to wound healing
PMNs are not essential to wound healing First infiltrating cells to enter the wound site, peaking at 24 to 48 hours
First infiltrating cells to enter the wound site, peaking at 24 to 48 hours Neutrophil migration is stimulated by
Neutrophil migration is stimulated by PMNs
PMNs Following functional activation neutrophils scavenge:
Following functional activation neutrophils scavenge: PMNs do not survive longer than 24 hours
PMNs do not survive longer than 24 hours Stimulated neutrophils
Stimulated neutrophils