Chapter 3
HEAD AND NECK RECONSTRUCTION
Head and neck reconstruction often refers to the aesthetic and functional rehabilitative efforts involving major traumatic and postsurgical oncologic defects. These reconstructive procedures may include replacement of lost tissues extending from the cutaneous to the mucosal regions of the head and neck including skin, muscle, neurovascular components, bone, and mucosal coverage. Challenges of head and neck reconstruction include but are not limited to restoration of the special senses in addition to voice, swallowing, speech, breathing and even the aesthetics of emotion in facial animation. This chapter discusses reconstruction and rehabilitation of the common postoncologic defects and is based on the most widely accepted state-of-the-art techniques for the specific recipient sites listed. It is not intended to be an all-inclusive, comprehensive review but rather a concise reference for those approaching the more common defects with a desire for the best functional and aesthetic outcome with the standard techniques currently used and available.
RECONSTRUCTION OF MAJOR HEAD AND NECK DEFECTS
Surgical reconstruction of head and neck defects originally consisted of second-intention healing, skin grafts, primary closure, and locoregional random flap reconstructions.1–3 It was not until the use of various neck and forehead flaps followed by the use of the deltopectoral flap that larger defects could be reconstructed or closed.4–9 Expanded use of these flaps exposed several problems including limited length, arc of rotation, and unreliability, whereas more reliable and lengthy regional flaps such as the pectoralis and other myocutaneous flaps became the mainstay for reconstruction of larger defects following cancer surgery.10–15 The innovative and customized techniques beginning with the pectoralis myocutaneous and osteocutaneous regional flaps provided the framework on which microvascular free tissue transfers were applied to the head and neck and the future of head and neck reconstruction was born.
ONCOLOGIC AND RECONSTRUCTIVE ISSUES
Reconstruction of the head and neck region must address a variety of functional and cosmetic areas that are apparent in public on a daily basis. Thus, the goals of head and neck reconstruction include a general plan to provide the least morbidity in returning patients to their pretreatment or original function and aesthetics. It should be mentioned at the outset that the curative intentions of a procedure should not be compromised to provide for a particular reconstructive option except under extenuating circumstances as agreed on by the treating and reconstructive teams with the patient and family. The primary goal, therefore, is to provide the patient with the best possible life expectancy through reconstruction aimed at also providing the best quality of life and patient satisfaction. In certain cases, this may include delayed reconstruction to provide the ability to “monitor” a wound for recurrent disease for a period of time prior to the definitive reconstruction. A second goal, yet often equally important, is to provide for functional breathing, chewing, speech, and swallowing by means of reconstructive opportunities. Further refinements in functional reconstruction should address mastication and dental rehabilitation, sensation, salivary flow, taste, and articulation, among others.
The cosmetic result remains a priority to the reconstructive surgeon but it should be weighed with the patient’s desires in relationship to the aforementioned goals. It is not always possible to provide the best cure rate, functional reconstructive option, and optimal aesthetic outcome with a single flap reconstruction. The patient and family should be well educated about these issues prior to surgery and about the expected outcomes, risks, complications, and options.
One of the obvious advances in head and neck cancer over the past half century has been the increasing variety of reconstructive options allowing surgical resection of larger tumors involving structures vital to functional and aesthetic rehabilitation. The current concept of reconstructing missing tissue with similar tissue and the potential for vascular and neural input allows patients improved quality of life and has provided patients with historically unresectable tumors (or “unreconstructable”) the option of surgical resection. In any reconstruction there are considerations of both donor site and recipient site defects, morbidity, and functional and aesthetic results. For cutaneous defects, the skin color and thickness are especially important, but with composite flaps in through-and-through defects the skin color match is not the top priority. For large mucosal defects, cosmesis is of less importance as function becomes the top priority. With all of these considerations, the head and neck reconstructive surgeon should remember that the type of reconstruction or combination of reconstructions affording the patient the best cosmetic result, quality of life, and least donor site morbidity is often the method of choice. The following surgical reconstructive techniques are divided into pedicled flap reconstruction and free tissue transfer reconstruction.
PREOPERATIVE PLANNING
It is critical for the ablative surgeon to communicate with the reconstructive surgeon about the planned surgical procedure, approach, and expected reconstruction well in advance of the procedure. The reconstructive surgeon may often order additional studies based on the location of the tumor, planned reconstruction, comorbidities, and patient occupation or avocation prior to surgery. Presence of adequate vascular structures in the neck may require confirmation by examination, ultrasound, arteriography, or computed tomography (CT) or magnetic resonance imaging (MRI) scanning prior to free tissue transfer. The numerous regional flaps based on vascular structures surrounding the head and neck regions require detailed knowledge about, and experience with, the vascularity of the region, whereas the expanding use of free tissue transfer demands meticulous dissection and care of these same vessels for recipient microvascular anastomosis. Although much focus is given to surgical techniques, consideration should always be given to prosthetic rehabilitation and emerging technologies. For example, a patient with an oral cancer may benefit from an intermediate or permanent obturator or stent through consultation with a maxillofacial prosthodontist in combination with surgical reconstruction. Many maxillary and palatal cancers can be rehabilitated with either surgical techniques or prosthetic appliances.
Patient positioning, planned incisions, preparation, and instrumentation are often modified according to the technique planned. Most major defects of the head and neck are ideally reconstructed at the time of surgical extirpation, which provides immediate functional and aesthetic improvement preventing extensive scarring, fibrosis, and other morbidities.
PEDICLED FLAPS
Pedicled flaps used in head and neck reconstruction commonly are used in a primary reconstruction, although delaying these flaps can provide additional length when necessary. The common flaps applied to this region are listed in Table 3-1 and are supplied by axial blood supply from the head, neck, or trunk in the majority of cases. These flaps are described with an emphasis on the more commonly used procedures.
FASCIOCUTANEOUS FLAPS
DELTOPECTORAL FLAP
Originally described by Bakamjian, who reported first using this technique in 1962, this flap remains an option for head and neck reconstruction, although its limited arc of rotation may limit its application to certain defects of the head and neck and pharynx unless delayed.6,16 The flap is a fasciocutaneous axial flap based on the first four intercostal perforators with the majority of the blood supply from the second and third branches of the internal mammary artery. The flap should be preserved in all patients undergoing a pectoralis myocutaneous (PMC) flap due to the potential division of the deltopectoral (DP) flap during incisions for exposure of the PMC flap. This provides a “backup flap” for any future reconstructive needs. It also provides excellent vascularized coverage of the peristomal and lower pharyngeal region as a one-stage axial flap when the flap does not extend beyond the DP groove. When an extended length flap is necessary, the flap usually requires a delay procedure 3 to 4 weeks prior to transfer of the extended flap into the deltoid region. With delay, the flap has been used to cover most regions of the head and neck. The major disadvantages of the DP flap are the limited arc of rotation and length of flap and the donor site defect, which can be unsightly with the skin graft coverage. When used for total pharyngeal defects, concerns include a high rate of stricture and fistula formation.
Technique
The flap should be outlined prior to any PMC flap harvest and it is recommended to outline the delayed extended DP flap in case additional tissue is necessary (Fig. 3-1). During the first stage, the flap is marked along the inferior border of the clavicle from the sternal notch to the acromioclavicular joint. It then takes a gentle curve well beyond the DP groove over the shoulder and inferiorly to 1 cm superior to the axillary crease to meet the inferior border along the fourth intercostal space to the sternum. An operative towel or sponge is used to provide a template to determine the arc and distal limits of the flap prior to the incisions, holding one end firm at the junction of the sternum and fourth intercostal space and rotating the towel toward the defect. If additional length is required, the incision can be extended at this time, or a back cut can be performed at the inferior aspect, taking care not to extend the cut to the depth of the vascular supply. The flap is then elevated from the distal tip to the DP groove in a subfascial plane. The flap is sutured back in position until the second stage 3 to 4 weeks later. At the second stage, the flap should have no separation or necrosis and can be elevated in the same plane to within 2 cm of the lateral edge of the sternum where the perforators can usually be visualized entering the flap. The flap is then transferred into position and sutured into place. The donor site is covered with a petrolatum-based gauze while the lateral half of the wound is covered with a split-thickness skin graft. The flap can then be released 3 to 4 weeks later if the base is to be returned to the donor site. Physical therapy is usually required to prevent significant fibrosis and scar contracture of the upper chest and shoulder.
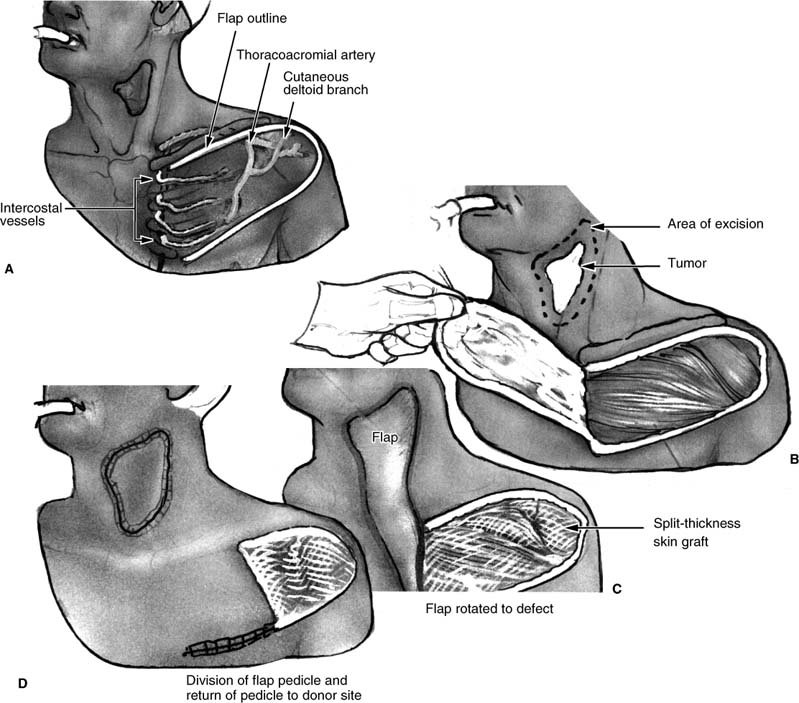
FIGURE 3-1 Deltopectoral fasciocutaneous pedicled flap. (A) Common placement of the deltopectoral incisions in relation to the vascular supply. (B) View of the cervical defect and deltopectoral flap elevation prior to transfer. (C) Transfer of the deltopectoral flap into the cervical wound prior to division of the pedicle. (D) Final view after division of the deltopectoral flap.
MYOCUTANEOUS FLAPS
PECTORALIS FLAP
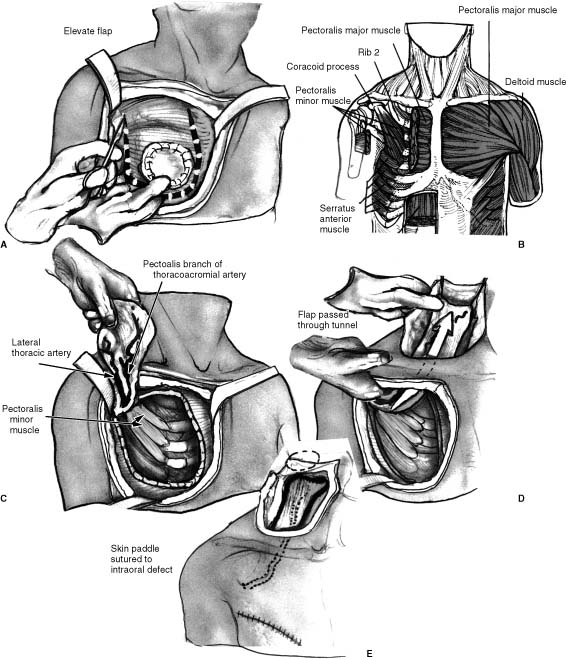
The pectoralis myocutaneous regional pedicled flap (PMC flap) was originally transferred for chest wall defects but widespread use in the head and neck region for a variety of defects resulted in head and neck applications outnumbering those for chest reconstruction.13,17,18 The PMC flap remains the workhorse flap for head and neck reconstruction more than two decades after it was given this title. The flap provides adequate, vascularized soft tissue coverage for many head and neck defects with minimal donor site morbidity and high success rates. Complete flap failure rates remain less than 3%; however, there is considerable evidence that partial failure, flap separation, dehiscence, or fistula formation occurs in up to 30% of cases.19 The flap remains limited in length by its vascular pedicle, although further lengthening can be performed by resecting a segment of clavicle. The PMC flap remains an excellent “backup” flap if tumor extirpative surgery results in a defect that cannot be closed primarily or for failure or less than ideal conditions with other reconstructive options. Outcomes analysis of PMC flaps versus other flaps are limited to specific areas and indications and cannot be generalized. It is obvious that the PMC flap is going to remain a reconstructive option in the head and neck for years if not decades to come.
Anatomic Considerations
The PMC flap is composed of (from deep to superficial) deep pectoral fascia, pectoralis major muscle, superficial pectoral fascia, adipose tissue, and skin. It is a fan-shaped muscle with origin from the medial half of the clavicle onto the sternum and cartilages of the second to sixth ribs. It courses superolaterally to insert into the crest of the greater tubercle of the humerus. It acts to flex, adduct, and rotate the arm and shoulder medially, thus revealing the importance of physical rehabilitation when this flap is used in reconstruction. The vascular supply is derived from the two major branches of the second part of the axillary artery, the thoracoacromial and lateral thoracic, although the major flow is through the pectoral branch of the thoracoacromial.20 It is supplied by the motor branches of the medial and lateral pectoral nerves from the brachial plexus with supply from the fifth to eighth cervical and first thoracic nerves. Other anatomic structures that should be well understood in harvesting the flap include the intercostal perforators to the chest and DP flap, the intercostal muscles, pleura, clavicle, anterior costal cartilage and ribs, rectus abdominis muscle, pectoralis minor muscle, and anterior serratus muscle.
Indications
The indications for the PMC flap include mucosal defects extending from the level of the soft palate anteriorly to the floor of mouth and inferiorly to the cervical esophagus. The PMC flap has the potential reach for skin and soft tissue defects from the periauricular region to the perioral region and the entire neck. An extended PMC flap with a random distal extension may reach to the temporal bone and maxillary region.
Advantages
The advantages of this flap include its reliability and source of vascularized tissue in an area adjacent to the head and neck but not exposed to treatment-related radiation therapy or prior scar from head and neck surgery. It provides skin, adipose, and muscle bulk that may result in thinner coverage from muscle atrophy when the pectoral nerves are divided at the time of harvest. Its application can be applied to most any defect in the head and neck region except for the most cranial regions due to its limited reach.
Disadvantages
The disadvantages of the flap are related to attempts to use it to replace bone or in upper pharyngeal and oral defects. It is insensate, and the thick skin and excess bulk may prevent a return to normal speech and swallowing. The flap is not the ideal flap for osseous defects but has been successfully used to fill in bone defects with or without plating in the oromandibular region. When used in total pharyngeal reconstruction, it does have a high rate of fistula and stricture formation compared with other techniques such as the jejunal free flap and tubed radial forearm free flap. Donor site morbidity includes significant scar and asymmetry to the chest wall and soft tissue bulging over the clavicle. Therefore, a surgeon should not consider it as the only flap to reconstruct head and neck defects. Careful patient selection does, in some cases, necessitate its use when microvascular reconstructions are not available or contraindicated. The arm and shoulder function may be affected particularly in patients undergoing extensive neck dissection or in those unable to complete proper physical therapy after surgery.
Technique
The flap is usually transferred as a myocutaneous flap, which is described here (Fig. 3-2). The expected recipient site is analyzed to determine the geographic relationship and route of transfer of the PMC flap, taking into account that the flap usually must run deep to the existing neck skin, further limiting its length. As with the DP flap, a gauze template is used to estimate the necessary length and arc of rotation, allowing for orientation of the skin paddle. Once the planned cutaneous flap measurements are available, they are marked in the inferomedial chest between the sternum and nipple with possible extensions into the abdomen or proximally in the chest. The incision is made through skin, subcutaneous tissue, and pectoralis muscle fascia. The fascia is secured to the dermis initially to decrease the risk of shearing of the flap from the muscular perforators. Wide undermining is performed circumferentially away from the paddle to provide exposure to the muscle and vascular pedicle and to facilitate eventual closure of the donor site, which rarely requires skin grafting. Superior undermining in the subfascial plane continues superficial to the clavicle to connect with the neck subplatysmal flap elevation. It is helpful to widen the tunnel to allow easy passage of the surgeon’s hand to prevent compression of the muscle and pedicle. The lateral border of the pectoralis major muscle is identified and elevated from the chest wall to visualize the vascular pedicle. The pectoral branch and the lateral thoracic artery can usually be identified, and, if possible, both are preserved. The muscle is then divided distal to the flap, which sometimes requires separation of a portion of the rectus abdominus muscle. The entire skin, adipose, and muscle are elevated from the chest wall to the pectoralis minor muscle and beyond to the level of the clavicle. Small intercostal perforators enter the muscle through the chest wall and should be ligated. The flap can then be tunneled into position by passing an Allis clamp and grasping the subcutaneous tissue pulling the flap into the neck and taking great care that the flap is not twisted. Closure of the chest wound is done with a two-layer closure, and a drain is placed inferiorly and laterally to prevent hematoma formation. The PMC flap is a heavy flap, and gravity tends to pull the flap inferiorly away from the suture line unless several “antigravity” sutures are placed. The pectoral nerve is divided to prevent muscle contraction and suture dehiscence in addition to potentiating muscle atrophy.
FIGURE 3-2 Pectoralis myocutaneous pedicled flap. (A) Placement of incisions for the pectoralis myocutaneous flap with skin elevation and planned muscle division incisions. (B) Anatomic structures deep to the pectoralis muscle on the right as viewed during elevation of the pectoralis myocutaneous flap. (C) View during elevation of the pectoralis myocutaneous flap from the anterior chest wall. (D) Tunneling of the flap deep to skin encompassed within the deltopectoral flap region into the neck. (E) Flap placement deep to the neck skin with the intraoral flap inset not visible.
LATISSIMUS DORSI FLAP
The latissimus dorsi myocutaneous flap (LDMF) is one of the few flaps that can be pedicled to reach almost any defect in the head and neck region. First described in 1896 by Tansini for chest wall reconstruction and developed for use in the head and neck by Quillen et al10 in 1978, it has been applied to defects of the scalp, maxillofacial region, skull base, mandible, and pharyngoesophagus.21–25
As a myocutaneous flap it can be used for skin coverage and mucosal coverage, whereas the myofascial flap is often used to add bulk, cover vascular structures, and cover skull base and scalp defects. It is not often the primary flap used in head and neck reconstruction due to the convenient transfer of other pedicled myocutaneous flaps such as the pectoralis major and free tissue transfer, which often allows more customized flaps for particular defects. It is more often used in secondary reconstructive procedures or in procedures requiring flap reconstruction of the lateral skull base and scalp due to its extended length and thin soft tissue coverage. It has more recently been transferred as a free tissue transfer, which avoids the transaxillary dissection and postoperative concerns over positioning of the arm.
Anatomic Considerations
The latissimus dorsi is a thin, triangular-shaped muscle that covers the lower half of the lumbar region extending laterally to the abdominal wall musculature. The vascular supply to the muscle is primarily from the thoracodorsal artery, which is paralleled by a larger vein; however, the intercostal and lumbar perforating vessels supply the lower portion of the muscle and are not harvested with the myocutaneous pedicled flap.26,27 The thoracodorsal artery is the terminal branch of the subscapular branch of the axillary artery and usually provides a pedicle 7 to 10 cm in length from the branching off of the subscapular artery. The artery typically varies from 2.0 to 4.5 mm in diameter. Distal branches may allow splitting of the flap into two paddles when inner and outer lining of head and neck defects is necessary.27 The thoracodorsal nerve from the posterior cord of the brachial plexus innervates the muscle, whereas the lateral cutaneous intercostal nerve supplies the skin of the region of the distal muscle where the cutaneous paddle is often harvested. The muscle originates in a broad aponeurosis, which attaches to the lower six thoracic vertebrae along with the lumbar and sacral vertebrae. It has numerous attachments including the lower ribs and the posterior iliac crest. From this fan shape, it extends superolaterally attaching by fibers to the tip of the scapula to insert in the intertubercular groove of the humerus. The latissimus muscle functions by pulling the shoulder downward and backward while adducting, extending, and rotating the arm medially with the help of the pectoralis major.
Indications and Preoperative Planning
The latissimus dorsi pedicled flap (LDPF) is most often used for reconstruction of lateral neck, parotid, and temporal bone defects. It may also be required secondarily following recurrent cancer surgery or prior flap failure when other donor sites are not available. Caution should be exercised in patients who have had a previous axillary dissection or radiation for melanoma or breast cancer. In these cases, arteriography may be indicated to determine the patency of the vascular supply. Prior pectoralis flap harvest on the ipsilateral side may complicate the axillary dissection when this flap is transferred as a pedicled flap and also result in significant debilitation to the arm and shoulder function. The muscle is often palpable, and planning of the location of the skin paddle can be determined by having patients flex their arms with their hands on their hips.
Technique
The dimensions of the muscle are outlined prior to marking the skin paddle. The anterior border of the muscle is palpable and runs from the axillary crease to the posterior superior iliac crest. Doppler flow can be tested to confirm the location of the vascular pedicle if necessary, although the pedicle runs a reliable course deep and medial to the lateral border of the muscle. The patient is often positioned in a lateral decubitus position with a beanbag supporting the body and an axillary roll supporting the axilla, preventing brachial plexus injury. The flap can be harvested with a two-team approach if the extirpative surgeon has access to the extirpative site and a contralateral neck dissection is not necessary. The ipsilateral arm is wrapped in a stockinette and no intravenous (IV) or arterial line is placed in this arm. The skin paddle should be placed within the middle third of the muscle due to a more random pattern blood supply distally
Flap Harvest
The initial incision surrounds the planned skin paddle and should include a curvilinear extension along the superior aspect of the muscle toward the axilla. Lateral to medial dissection exposes the lateral border of the latissimus muscle and the vascular branches to the serratus anterior muscle, which, if followed, branch with the thoracodorsal vessels. Once these are identified, the branches to the serratus are ligated and the lateral to medial flap elevation from the thoracic wall is performed. The thoracodorsal vessels can then be dissected proximally into the axilla, identifying the angular artery to the tip of the scapula, the circumflex scapular artery, and the branch to the teres major muscle. This is facilitated by division of the muscle from the tip of the scapula, enhancing exposure. Once the vascular pedicle is identified and exposed, the entire muscle and skin paddle are dissected free distally to proximally For the pedicled flap, a tunnel is then made between the pectoralis minor and major through which the flap is gently passed into the upper chest and neck region. Alternatively, depending on flap compression and prior surgical procedures, it can be tunneled subcutaneously through the axilla. During and following the procedure, the pedicled flap can be compressed by the arm and shoulder, causing thrombosis if it is not monitored carefully and arm position changed as necessary. The arm should be immobilized until flap viability and healing is assured. Any change in color requires evaluation by the surgical team. This flap has also been transferred with attached ninth rib as an osteomyocutaneous flap for mandible reconstruction, although the vascularity and eventual resorption of the bone can be a problem. As a free flap transfer, it is often combined with one of the variations of the scapula flap to include bone and adjacent skin paddles.
Advantages
The latissimus flap has an extremely long pedicle and transfer capability to most regions of the head and neck. It is reliable but requires intense monitoring to prevent thrombosis. In a large series of 68 cases over a 14-year period, Hayden et al24 report only one case of total flap necrosis and six cases of partial flap necrosis. The flap provides a large surface area of thin cutaneous covering or vascularized tissue for soft tissue defects. It is the ideal flap for total scalp defects and provides nice shape and contour in radial parotidectomy and lateral temporal bone resections. It has minimal donor site morbidity that is usually imporved with physical therapy.
Disadvantages
The major disadvantage is the need to modify the patient position intraoperatively or perform the procedure with the patient in the lateral decubitus position. Seroma formation represents one of the more common complications at the donor site.
TRAPEZIUS FLAP SYSTEM
The trapezius system of flaps has been described by several authors for a variety of soft tissue and osteocutaneous defects in the head and neck region.12,13,28–30 The trapezius muscle is the central component in several flaps named the superior, lateral island, and lower trapezius flaps. These flaps are infrequently used relative to other regional flaps but are most applicable to defects of the lateral neck, lateral skull base, scalp, and maxillofacial region. The lateral island trapezius flap has been transferred with bone for mandibular and maxillofacial reconstruction.
SUPERIOR TRAPEZIUS FLAP
The superior trapezius flap is based primarily on the posterior superior intercostal perforating arteries with contributions from the occipital artery, and is the most convenient to use for head and neck reconstruction due to its position along the posterior neck and shoulder. Similar to the DP flap, it may require delay for extended length flaps to reach the anterior neck, oral cavity, and pharynx. It is limited by its arc of rotation in the posterior midline and usually requires a skin graft for closure of the donor site.
LATERAL ISLAND TRAPEZIUS FLAP
The lateral island trapezius flap is based on the transverse cervical artery and requires extensive dissection of this vessel into the neck to provide adequate mobility for rotation into more anterior regions of the head and neck. Its vascular supply may be tenuous in individuals previously treated with surgery or radiation to the neck region, and this represents a relative contraindication to the procedure. Both the superior and lateral island trapezius flaps can be performed with the patient in a semilateral decubitus position, whereas the lower trapezius flap must be done with complete exposure of the patient’s lower back to the midline.
LOWER TRAPEZIUS FLAP
The lower trapezius flap is the most difficult to harvest of the three trapezius-based flaps but provides the most tissue that can be rotated to a wider array of recipient sites than superior and lateral flaps. Based on the deep branch of the transverse cervical often referred to as the dorsal scapular artery, this flap has less consistent vascular anatomy, and meticulous dissection of these distal branches of the transverse cervical vessels is required. The skin paddle is centered between the tip of the scapula and vertebral bodies in the mid-back, and a curvilinear incision is extended superiorly to identify the supplying vessels. The donor site can often be closed primarily with adequate drain placement prior to closure to prevent seroma formation.
TECHNIQUE
The harvest of these flaps generally includes the skin, subcutaneous tissue, and trapezius muscle, along with meticulous technique in identifying and preserving the vascular pedicle. These flaps can be applied primarily to posterior neck, lateral neck, and temporal bone defects, although other flap options are typically preferred. Detailed information about the anatomy of the vascular supply and the flap harvest technique are well described in prior reports.30–32 Technical preparation is required prior to undertaking this flap harvest.
PLATYSMA FLAP
The platysma flap is a myocutaneous flap that is based on branches of the facial artery and can include venous drainage from the branches of the facial vein and external jugular vein.33 The flap should be planned prior to other neck surgery when the vasculature remains intact. Detailed consideration and planning is mandatory prior to placement of the neck incisions to provide for harvest and closure of the flap and donor site region. The major advantage of the flap is that it can be harvested near the field of a cancer resection, and it provides thin, mobile myocutaneous coverage for oral and pharyngeal defects. Although the flap is primarily supplied by the facial artery, it has been harvested successfully following ligation of this artery during radical neck dissection. Although most commonly applied to the oral cavity region, it has been used in numerous defects of the head and neck region.34–40
The primary disadvantage is that there is a high rate of partial flap necrosis with venous congestion and epidermolysis. Although the skin paddle often has partial necrosis, there is a low fistula rate when used for mucosal coverage.41
The flap technique requires that the distal flap incision be performed initially with a subplatysmal flap elevation, taking care not to injure the arterial and venous branches from the facial vessels. The proximal incision around the elliptical cutaneous paddle in the lower neck is elevated in a supraplatysmal plane, leaving an intact layer of platysma into the paddle. Modifications of this technique have been described that provide alternatives to flap harvest and reconstruction for appropriate defects.42,43 The flap is not usually the technique of choice when previous neck dissection or neck irradiation has been performed.41
SUBMENTAL FLAP
The submental flap is discussed in this section on regional pedicled flaps due to its potential for application in head and neck cancer reconstruction and because it is an axial flap based on the submental artery and vein. The flap has been reported to have a wide variety of uses in the head and neck region, including use in the oral cavity, lip, maxillofacial region, cervical esophagus, and for hemilaryngectomy defects.44–47 Anatomic studies have confirmed the vascular supply and caliber of vessels available for utility in adjacent regions.48–50
The flap can be harvested as a myocutaneous flap to include the digastric muscle or as a fascial or fasciocutaneous flap. It has the obvious advantage of being convenient to the head and neck region with excellent color match. The scar is usually hidden inferior to the mandible and provides an improved cervicomental angle in most cases. The major argument against the use of this flap is that inadvertent harvesting of lymphatics of level IA during the flap harvest may compromise lymphadenectomy and allow for locoregional recurrence in squamous cell carcinoma. In these cases, a thinned flap is practical to prevent the inclusion of lymphatics in the flap.
OTHER FLAPS
Many other local and regional pedicled flaps are used for coverage of defects of the head and neck, including nasolabial, cervical, cervicofacial, superficial temporoparietal fascia, forehead, and chest wall flaps, but a detailed review of all flaps is beyond the scope of this chapter.
FREE TISSUE TRANSFER
Head and neck reconstruction cannot be described in the 21st century without including free tissue transfer as an integral component in the current standard of care for various traumatic, oncologic, and congenital defects of the head and neck region. Few would disagree that free flaps have resulted in improved functional and cosmetic results of many head and neck defects, particularly the anterior mandible. It is important to understand that the reconstructive option for a particular defect is not a decision between a “free flap” or a “regional flap” or “primary closure”; instead it is a consideration of which individual flap, technique, or combination of procedures will afford the patient the optimal survival, quality of life, and cosmetic result. One of the obvious advantages of free tissue transfer is that it enables the surgeon to choose from a variety of tissues and flaps, thus tailoring the procedure to the patient. For example, flaps can be harvested with a variety of quantities and qualities of skin, fascia, adipose, muscle, and bone, and some advanced techniques allow neural input or motor input to provide sensate and motorized flaps for particular defects. Free tissue transfer requires a dedicated team of surgeons to successfully coordinate the extirpative procedure with the reconstruction, which often encompasses preparation of the donor site, recipient vessels, and insetting/pedicle geometry planning. Preoperative evaluation not only mandates a detailed history to assess prior trauma, surgery, or peripheral vascular disease, but also may require Doppler examinations, x-rays, or arteriography prior to flap harvest to determine the potential application of a particular donor site.
The flap failure rates for free tissue transfer continues to decrease with rates >95% despite such factors as exposure to mucosal sites, contamination, radiated tissues, and elderly patients. This may be attributed to an increase in the number and quality of centers with additional training dedicated to these techniques.51–56
Postsurgical monitoring and medical management of free tissue transfers remain controversial but include the use of a wide range of monitoring protocols and devices to assess clinical indicators such as flap color, temperature, texture, bleeding, and technologic advances including implantable and color Doppler.
This section reviews each of the more commonly used free tissue transfers for the head and neck region and discusses the technical considerations involving harvest and insetting of the more commonly used flaps. The text is organized by flap rather than by defect, thus allowing the reader to focus on the issues surrounding each individual flap. The most common flaps used in the head and neck region are listed in Table 3-2.
PREOPERATIVE CONSIDERATIONS
The analysis of the recipient site should address the type of tissues lost, functional needs, cosmetic issues, and availability of donor vessels of sufficient caliber and length, therefore directing the surgeon to the most applicable donor tissue. The patient’s occupation, avocations, and medical history along with the donor site morbidity further guide the surgeon to the best possible donor flap available.51,54
Although there are no general contraindications to free flaps, prior surgery and medical problems in specific areas may provide a contraindication to specific sites. Medical problems such as poor nutrition, diabetes, and coagulopathy may provide relative contraindications to certain procedures.
TIMING
Most head and neck free tissue transfers are performed primarily at the time of oncologic resection to provide separation from contaminated cavities, protection of vascular structures, and immediate functional rehabilitation.57 Secondary reconstruction in these cases often results in increased complications, scarring, nerve injury, and difficulty in the approach and insetting. Functional outcomes have also been shown to be poor following delayed reconstruction.58–60 In traumatic injuries and revision reconstructions, delayed reconstruction may provide an opportunity for improved outcomes after wounds have healed.61 Surgery and reconstruction following chemotherapy or radiation therapy add complexity and concerns when choosing free tissue transfer versus regional flap reconstruction.62 When additional reconstruction or secondary free flaps are necessary for recurrent cancer, postchemoradiotherapy failed free flaps, or cosmetic/functional indications, additional problems including approach, and microvascular anastomotic, donor, and recipient site issues must be addressed.25,59,60,62–65
OTHER POSITIONING
General inhalational anesthesia may be given via tracheotomy or orotracheal or nasotracheal intubation depending on the postoperative expectations for the patient’s function and edema. A beanbag, axillary roll, and arm rest may be necessary in the patient undergoing scapula, trapezius, or latissimus flap harvest. The author suggests that the anesthesiologist be positioned at the foot of the bed, which then allows adequate surgical exposure to the flap harvest and head and neck region with the operative microscope in place. The two-team approach should be considered to decrease operative time and improve patient outcome when possible.
INTRAOPERATIVE CONSIDERATIONS
The care provided to the head and neck free flap patient from admission to the hospital through discharge requires extensive communication and attention to detail. The staff in the preoperative holding area should be knowledgeable about the planned procedure to prevent accidental needle sticks, intravenous line placement, or arterial line placement in the planned donor site region.66 Patient position is dependent on the location of the flap and coordination of the procedure among the extirpative and reconstructive team regarding timing of each portion of the procedure. The position for harvest of flaps is discussed below. Coordination with the anesthesiologist should allow for timely administration of medications, the prevention of hypotension and hypothermia, and the use of various vasoconstricting agents during the procedure.
INTRAOPERATIVE MEDICATIONS
A variety of intravenous and topical agents have been advocated to improve flap viability, although widespread acceptance of any of these medications has not been achieved. Intravenous medications commonly include dextran, antibiotics, heparin, and in some cases steroids to reduce edema. Topical solutions and irrigants for use within and around the vascular pedicle and anastomosis have been advocated by many surgeons and include plain lidocaine or papaverine for improve vasodilation and prevention of vasospasm, and heparinized saline or lactated Ringer’s for irrigation.67,68
In certain situations, thrombolytic therapy including the use of streptokinase or urokinase69,70 may be considered to prevent microvascular thrombosis.71–74
ANASTOMOTIC ISSUES
Meticulous technique using fine microvascular instrumentations and 9-0 or 10-0 nylon suture is the common approach to microvascular anastomosis, although some authors now recommend the use of microvascular couplers for appropriate venous anastomosis.75–77 Preoperative understanding of the recipient and donor vascular anatomy, geometry, and size results in a pedicle with similar size match, tension-free geometric placement, and controlled anastomotic technique. The anastomosis is performed with running or interrupted suture in most instances, although a variety of technical modifications including the use of fibrin glue have been advocated.74,78–82
Meticulous techniques in handling of the vessels and branches while avoiding intimal injury allows for a higher success rate. Flap ischemia time should be minimized whenever possible, although studies have yet to confirm a maximum ischemia time for head and neck free flaps; a maximum 3-hour ischemia time is the goal for many surgeons.83,84 The timing and order of the anastomosis and insetting varies as some surgeons prefer to complete the anastomosis prior to insetting to decrease ischemia time, whereas others inset the flap initially, hoping to prevent manipulation and trauma to the anastomosis during the insetting. Consideration should be given to both options depending on the situation during the procedure. The author prefers to inset those flaps that require considerable movement during insetting such as osseous flaps and tubed forearm and jejunal flaps prior to completing the anastomosis. However, flaps that do not require excessive manipulation are usually secured to the recipient site or tunneled into position but not inset until the anastomosis is completed. Following completion of the procedure, suction drainage should be secured well away from the vessels to avoid trauma during patient movement.
POSTOPERATIVE CARE
Postoperative care should include close monitoring by the nursing staff to ensure a warm room, adequate oxygenation, positioning, and patient comfort. It is common to elevate the head of the bed to 30 degrees to prevent venous congestion. In hypotensive states, inflow problems may be more common, during which the head should be lowered to provide improved blood supply.
MONITORING
The survival rate of free tissue transfer has continued to improve over the past few decades, raising the issue of the necessity for close monitoring of all free tissue transfers. These high success rates are reported commonly from well-trained, experienced microvascular surgeons, emphasizing the importance of experience and technique.85 However, it is well known that in the small percentage of flap failures or thrombotic events, early recognition of the problem can prevent flap loss.51,54,86
The vast majority of flap failures and thromboses occur within the first 48 hours of surgery, and evidence suggests that bone containing flaps and flaps requiring vein grafts have a higher failure rate.86–88 Those flaps that develop signs of failure with thrombosis are most commonly due to a venous problem.88 There is no substitute for the meticulous technique in the handling of the vascular pedicle during harvest, transfer, insetting, and anastomosis. This section discusses important variables that should be considered in the perioperative care of patients undergoing free tissue transfer to prevent flap loss. The primary variables that may be used in flap monitoring all are directly or indirectly dependent on perfusion, including temperature, oxygenation, arterial supply, venous outflow, nutrition, and thrombotic events.
Prior to surgery, it is important to identify any past medical problems that may be related to the potential for a hypotensive episode, coagulopathy, thrombosis, infection, low oxygenation, or vasospasm.
A wide variety of monitoring techniques are presently in use and vary by surgeon and by specialty but include designing a “monitor” paddle, visual examination for color, continuous temperature and oxygen monitoring, laser and ultrasound Doppler scanning, photo and digital plethysmography, radioisotope clearance assays, and fluorescein perfusion monitoring.86,89–96
Regardless of the techniques used to monitor free flaps, the location of the recipient site flap, insetting issues, pedicle geometry, and tissue characteristics all play a role in determine the ideal type of monitoring.
VISUAL EXAMINATION
When flaps with a cutaneous or mucosal component are used, visual examination may be the best and most cost-efficient method to monitor the flap viability. Additionally, training nurses and physicians to identify early problems often requires that they have extensive experience to recognize subtle changes during visual inspection. The flap color is typically the same as the donor site from which it was harvested and may be paler or darker than the surrounding tissue at the recipient site. A change in color is important to note, as this may be the first sign of an arterial or venous problem. Capillary refill should be assessed and documented, with each examination noting any change or regional differences in the flap. Temperature, color, skin turgor, and texture of the flap often give early signs when a problem may arise. An arterial problem is often manifested by pale color, slow capillary refill, decreased thickness or decreased edema, poor skin turgor, softening, cool temperature and lack of bleeding when punctured with a 20-gauge needle. A venous outflow problem can be identified when the flap appears red, blue, or purple with brisk or immediate capillary refill, increased edema and firmness, and dark blood when punctured. When using surface Doppler assessment, it is important to consider adjacent or deep vessels, which may be transmitted through a thin flap to the Doppler, providing a false-positive examination for blood flow. Any change in surrounding tissues such as hematoma, wound dehiscence, salivary drainage, or infection should alert the surgeon that open exploration may be indicated.
BURIED FLAPS
Buried flaps represent a challenge for postoperative monitoring that has been addressed in a variety of methods by surgeons.54,91,92,97–99 When a flap is buried such as a jejunal flap, tubed radial forearm flap, or osseous flap without a skin paddle, assessment is often difficult and unreliable. If the flap is in the oropharynx, examination may be performed to view the flap. Otherwise, a natural external tissue “monitor” may be brought out into the skin to allow a way to directly monitor the flap. If this is not possible, direct monitoring through a buried probe may be helpful. When a problem is considered, early exploration for thrombosis is particularly useful to prevent complete flap loss.
FLAP THROMBOSIS AND SALVAGE
Although some surgeons do not monitor flaps closely due to greater than 95% flap success, those cases that are recognized within 4 to 6 hours following the onset of thrombosis have a 69% salvage rate when appropriate revision is performed.55 Studies have revealed venous thrombosis to be more common than either arterial or combined arterial and venous thrombosis. Over 75% of flap thromboses have occurred within the first 48 hours following completion of the procedure.86–88 Once the thrombosis is recognized, the patient should first have a complete evaluation to rule out nontechnical etiologies such as positioning, temperature, blood pressure, and hematoma. Correcting these problems at the bedside is done immediately while the operating room and personnel are alerted about the planned transfer to surgery. Medical therapies for the prevention and treatment of thrombotic events during free flap transfers often depend on surgeon preference, although some studies have revealed success with any one of a variety of protocols.55,69,71–73,100–103
The patient is usually administered intravenous heparin at 5000 U, and the neck is explored with gentle irrigation to view the vascular pedicle. Doppler examination of all arterial and venous structures should aid in the localization of the problem. Commonly, a venous thrombosis is identified, and this anastomosis is resected with fine microscissors, allowing decompression of the venous congestion. Often, the arterial flow then clears much of the thrombus, although any remaining thrombus may require clearing with a No. 2 or 3 Fogarty catheter. If further arterial problems are identified, the arterial anastomosis and inflow should be taken down to analyze the location or extent of thrombus. If necessary, arterial irrigation with streptokinase or urokinase at a dose of 50,000 to 100,000 U is performed. If another recipient artery is necessary, this dissection is performed, and if added length is required for the artery or vein, a saphenous vein graft is harvested. Confirmation of flow through the system is confirmed with heparinized saline irrigation using a 20-gauge angiocatheter. We withhold the use of leech therapy unless we have confirmed adequate arterial inflow but poor venous outflow. Complete venous obstruction mandates microvascular revision when possible, whereas partial venous congestion may be successfully treated in certain instances by leech therapy72,104–109 The mechanism of action of leeches is based on the agent hirudin, a selective thrombin inhibitor and potent anticoagulant.110 It is important to use prophylactic antibiotics due to the transmission of Aeromonas hydrophila by some leeches.105,108 When leeches are used in the head and neck, an adhesive covering is placed around the flap to prevent leeches from moving to other sites and constant nursing care is necessary to prevent movement into the pharynx or tracheotomy.
RADIAL FOREARM FASCIOCUTANEOUS FLAP
The utility and application of the radial forearm fasciocutaneous free flap in the head and neck has continued to increase since its introduction in the late 1970s.111–114 Its use was popularized by Soutar in the early 1980s, including the use of the osteocutaneous extension of this flap.113–117
It has wide application in the head and neck region, particularly in the aerodigestive tract. It has become the workhorse flap for head and neck reconstruction. and it is utilized more than any other single flap in two large retrospective reviews.52,118 Other authors have considered the lateral thigh flap to provide similar coverage of soft tissue defects.119,120 The ulnar artery-based forearm flap also is used by several surgeons with successful results for similar recipient site defects.121–123
Indications
The radial forearm flap is one of the most commonly used free flaps in head and neck reconstruction, particularly in the oral cavity, oropharynx, hypopharynx, and esophagus. The potential addition of bone provides anatomic reconstruction of areas requiring thin bone such as the ascending ramus of the mandible, alveolar arches, orbital rim, and zygomatic arch. The most common use of the radial forearm flap is in the oral cavity and pharynx, although it has enjoyed widespread use throughout the head and neck region including the skull base, trachea, partial laryngectomy defects, and maxillofacial defects.124–132
The radial forearm fasciocutaneous flap is ideally suited for partial to near-total glossectomy defects due to the thin, pliable, sensate skin covering. The flap usually mucosalizes over a period of 3 to 12 months, making it difficult to differentiate from surrounding tongue surface, and it also may regenerate sensate potential with or sometimes without nerve grafting.124,133,134 It can provide vascularized covering to most soft tissue defects of the oral cavity including total upper or lower lip defects, which can incorporate the palmaris longus tendon for lip suspension. It remains our flap of choice for large oropharyngeal defects, which include the pharyngeal wall, tongue, or soft palate due to the three-dimensional shaping required for these areas and its sensate potential. Its use in oromandibular reconstruction with lower donor site morbidity than initially reported may provide a benefit over other reconstructive options when osseointegrated implants are not indicated.135–139 Another common use is in total pharyngoesophageal defects for which it is useful as an alternative to the jejunal free flap.97,140–142 The ability to tube the radial forearm flap while in situ in the forearm can also decrease ischemia time.
Anatomic Considerations
As in all flaps to be discussed, the anatomy of the region should be studied by the surgeon in detail in anatomic textbooks, in cadaveric dissections, and by assisting in the harvest prior to undertaking free tissue harvest. Knowledge about the vascular structures proximal and distal to this area is important in assessing options for harvest and flow requirements for the flap. The radial forearm flap is based on the radial artery, which arises from the brachial artery near the antecubital fossa. The ulnar artery also arises in this region, and the relationship of the radial artery to the ulnar artery and radial recurrent artery is important during the harvest of both radial and ulnar-based flaps. Distally, the ulnar artery runs deep to the flexor carpi ulnaris but may be identified superficial in the distal forearm. The vessels usually communicate via the palmar arches in the hand, and if there is not a patent arch, the flap is contraindicated without a vascular graft. The skin, bone, and fascia of the flap are supplied via perforators easily identified during the dissection. The deep periosteal perforators extend through the flexor pollicis longus muscle to the radius. During the dissection, the anatomic location of the radial and ulnar arteries, superficial veins and vena comitantes, flexor carpi radialis, brachioradialis and palmaris longus tendons and muscles, and the radial and median nerves should be appreciated. During the harvest of bone, the insertion of the brachioradialis tendon is the distal extent of bone harvest.
Advantages and Disadvantages
The radial forearm flap has numerous advantages and few disadvantages, although in some patients the disadvantages may outweigh the potential advantages. The flap is extremely reliable and has a consistent pedicle that is lengthy and of large caliber.52,143,144 It is thin and liable with sensate potential, adding to the benefit in individuals with expected return of swallowing function. It may be harvested by two teams and is easily contoured and shaped to a particular defect. It is well vascularized throughout its length, allowing for the use of split-paddle or even three-paddle skin coverage. The potential addition of bone provides anatomic reconstruction of areas requiring thin bone such as the ascending ramus of the mandible, alveolar arches, orbital rim, and zygomatic arch. The skin color match is fair at best in Caucasians but good to excellent in the non-Caucasian population. The donor site remains the major disadvantage of the use of this flap, although specialized techniques may improve on the unsightly scar and resulting morbidity of this site.145–147 If aggressive physical therapy is not instituted, limitation in range of motion of the wrist can occur due to scarring and fibrosis.
Preoperative Evaluation
The anatomic dissection of the soft tissue harvest of the radial and ulnar flaps overlaps considerably, so this discussion will focus on the neurovascular, soft tissue, and bony landmarks of the volar aspect of the distal half of the arm. Unlike many donor sites, the majority of the structures of the arm are readily visible or palpable prior to the surgical harvest. It is important to assess the skin of the entire arm from the shoulder to the tips of the fingers for prior surgical or vascular intervention. Any compromise of vascular flow, including prior blood draws and needle sticks, can interfere with the viability of flaps of this region. The gold standard test prior to harvest is Allen’s test, which is performed to assess the patency of connection of the superficial and deep palmar arches in the hand.148 The test is performed by asking the patient to firmly make a fist repeatedly while the examiner is simultaneously compressing the radial and ulnar arteries. After complete blanching of the hand is confirmed despite the patient’s cessation of making a fist, the ulnar artery compression is released while firm pressure remains on the radial artery (thus duplicating the effect following ligation of the vessel). The return of blood flow to the digits, particularly the fourth and fifth, is assessed to visualize rapid (less than 5 seconds) return of flow, confirming patency of the two arches. If repeated tests indicate a problem with the patency, the other arm should be examined, and Doppler studies should be considered to fully evaluate the system prior to harvest. There have been rare cases of anomalous forearm vascular supply, which must be considered prior to the radial forearm harvest to prevent ischemia to the hand.143,149,150 The brachioradialis, flexor carpi radialis (FCR), palmaris longus, radial nerve, radial artery pulse, capillary refill, antecubital fossa, and cephalic and basilic veins should all be examined and identified prior to the planned donor site harvest.
Techniques
Harvest
The arm should be free of intravenous or arterial lines and needle sticks. A second nursing and surgical team is necessary for the two-team approach. Prior to incisions, the arm is marked for important landmarks including the radial artery, superficial veins, and radial nerves. A tourniquet is applied to the upper arm but is not inflated until the arm is exsanguinated and the procedure is ready to begin. The arm is extended on an armboard, allowing the surgeons to sit opposite each other during the dissection. The thigh should be prepared or appropriate allograft material available for coverage of the donor site. The planned skin paddle is marked overlying the volar aspect of the forearm centered on the donor artery. A curvilinear incision extends to the antecubital fossa for extended length pedicle. When the necessary cutaneous paddle may be positioned away from the bone during the osteocutaneous transfer, the skin paddle may be located toward the ulnar aspect to facilitate three-dimensional mobility.
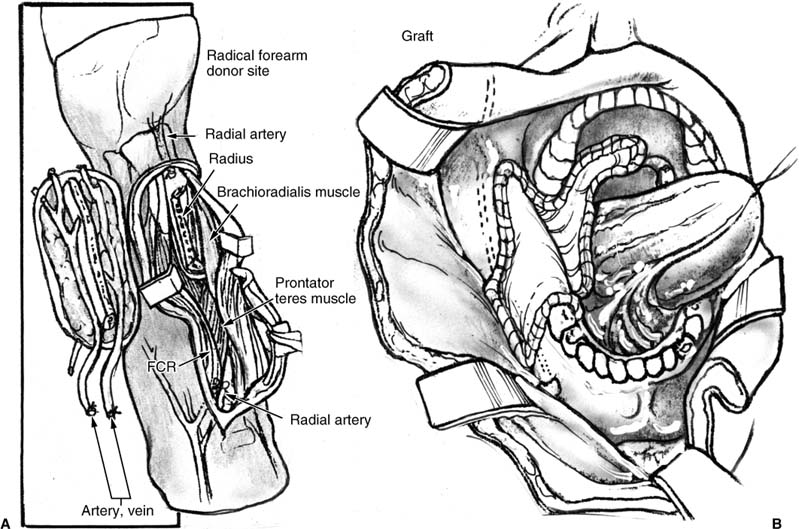
FIGURE 3-3 Radial forearm osteofasciocutaneous free flap. (A) Illustration of the donor site flap elevation. (B) Insetting of the radial forearm fasciocutaneous free flap for defect of the lateral mandibular and oropharyngeal soft tissue defect.
The skin and underlying fascia are then elevated from the radial aspect toward the ulnar aspect, identifying the distal radial nerve branches that may divide into two to five small branches at the wrist. Care is taken to divide the fascia at the brachioradialis and dissect deep to the vascular pedicle, preventing separation of the cutaneous paddle if dissection occurs superficially. After the pedicle is identified, the venous branches of the superficial and deep system are ligated, leaving the radial artery flowing distally beyond the flap. The dissection is then started from the ulnar aspect and elevated toward the pedicle, taking care to remain superficial to the ulnar artery and median nerve. Important points to remember during the harvest are to preserve the paratenon fascia overlying the tendons of the flexor carpi radialis, palmaris longus, and brachioradialis muscles to prevent tendon exposure. The dissection then proceeds deeper when the lateral edge of the FCR is identified, taking care to identify the deep perforating branches to the periosteum of the radius. The deep osseous branches are ligated when bone is not harvested and carefully preserved when an osteocutaneous flap is harvested. The distal and proximal incisions are connected around the periphery of the flap, leaving the flap connected proximally through the neurovascular attachments and by the radial artery distally. The cutaneous nerves are immediately deep to the subcutaneous tissue proximally and should be preserved when possible. These nerves usually run alongside the distal branches of the superficial venous system. At this point, separation of the brachioradialis and FCR allows visualization of the proximal radial artery and paired venae comitantes to the antecubital fossa. The deep and superficial systems merge into a plexus of veins in the antecubital fossa and beyond, often allowing the surgeon to perform fewer venous anastomoses but allowing flow through both systems.
This section describes variations that apply exclusively to the osteocutaneous flap harvest (Fig. 3-3).
The flexor digitorum superficialis (FDS) must then be separated from the radius distally to provide exposure to divide the flexor pollicis longus (FPL) and periosteum of the medial aspect of the radius. It is recommended that less than 40% of the diameter of the radius be harvested, and the length should be limited to the bone distal to the insertion of the pronator teres and proximal to the insertion of the brachioradialis. Studies have supported the use of internal rigid fixation to prevent radius fracture and improve the strength of the remaining radius, thus requiring preservation of a segment of distal radius for screw fixation. The bone is exposed along the length of the harvest site, and after elevating the periosteum at the planned osteotomy sites, the oscillating saw is used to divide the partial-thickness proximally and distally. The osteotomy along the length of the radius is then performed carefully to avoid overresection. The flap is ready for transfer. After transfer, hemostasis is obtained and the plating of the radius is performed under fluoroscopic confirmation of plate placement. The procedure is completed as described in the standard fasciocutaneous technique.
The distal radial artery is then clamped with a temporary clamp while the tourniquet is released, confirming flow to the digits through the palmar arches. It is then safe to ligate the distal radial artery and transfer the flap when the recipient site is prepared. The forearm should be closed with a split-thickness skin graft, although a recent study suggests that allograft material may be a suitable replacement.151 A prefabricated forearm splint is placed to secure the graft material for 7 days, and monitoring of the vascular supply to the hand continues throughout the postoperative period.
The most common complications following radial forearm flap harvest include skin graft loss, sensory deficits, and cold intolerance and motor strength deficits of the hand.152–155 The paratenon fascia preservation overlying the tendons cannot be overemphasized, as this is the enveloping fascia that allows vascular ingrowth from adjacent tissues and grafts.156 When this is removed, it is helpful to advance adjacent muscle or adipose to cover any exposed tendon prior to grafting.
Physical therapy with wrist and hand range-of-motion exercises should commence within weeks following the procedure to ensure adequate motor function.
The three to five small, superficial branches of the radial nerve should be identified early in the dissection to prevent accidental transection or trauma and permanent numbness, although some patients do have resultant sensory loss when these branches are preserved.154
The contraindications to the radial forearm flap include generalized coagulopathic states and other medical conditions that prevent all free tissue transfers. The unique contraindications include anomalous palmar arch anatomy, radial or ulnar agenesis or thrombosis, and prior surgery or trauma to the forearm area. Patient function, occupation, and avocation may preclude the use of this donor site. The flap has been successfully transferred by the author in patients with connective tissue disorders and graft-versus-host disease.
CONTROVERSY
Preoperative evaluation has been discussed by various authors and has consisted of Allen’s test alone, preoperative Doppler assessment, or intraoperative monitoring with assessment of distal flow to the digits.143,148–150,157 The author recommends Allen’s test in all patients and Doppler assessment in those with an equivocal Allen’s test or prior arterial line placement. Post-harvest grafting techniques have included split-thickness skin graft, dermal graft, pursestring with grafting, local flaps, and allograft materials.151 Controversy exists about the use and technique of various flap and graft materials including the prefabricated fascial radial forearm free flap to preserve donor site cosmesis and tendon coverage.158–160
The use of the osteocutaneous radial forearm flap has not gained wide acceptance due to early reports of increased donor site morbidity including fracture of the radius. The lack of adequate bone for osseointegrated implants is a second reason for its limited use in mandible reconstruction, although limited donor morbidity and reduced fracture rate have been reported.135,136
FIBULA OSTEOCUTANEOUS FREE FLAP
The fibula free flap has become the gold standard for reconstructive procedures of large mandibular defects owing to its excellent bone stock, skin paddle availability, sensory potential, and acceptance of osseointegrated implants. Initially described by Taylor, it was applied to the mandible and popularized by Hidalgo and others for head and neck reconstruction.161–165 The early application of the fibula osteocutaneous flap revealed a high failure of the cutaneous paddle due to variations in the septocutaneous and musculocutaneous perforators and the early loss of the skin paddle during mandibular reconstruction. The flap has since been introduced with a variety of modifications using osteotomies and sensory innervation and applied to numerous regions of the head and neck.90,165–169
Indications
The fibula free flap is most commonly used as an osteocutaneous free tissue transfer that is ideal for reconstructing oromandibular defects, although utility in the orbitomaxillary regions has also been successful. It is the flap of choice in composite segmental oromandibular defects of the anterolateral mandible with soft tissue loss when osseointegrated implants or sensory innervation would benefit the patient. It is also useful in reconstructing oromaxillary and orbitomaxillary defects, providing excellent bone stock, which permits osteotomies for accurate contouring and insetting170
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree