Fig. 12.1
Fetal development of the hair follicle
During the second quarter, the peripheral epithelial cells are separated from the central epithelial cells in the hair bulb, thus forming the outer root sheath. The centrally located cells are situated above the dermal papilla and are later differentiated in the inner root sheath and in the hair shaft with its cuticle, cortex, and medulla. All hair follicles are formed during the period of embryonic life, and in humans additional follicles are not formed after birth. The maximum density of hair follicles on the scalp is found in newborns and gradually decreases with age until adulthood [2]. There are two types of hair: (1) the vellus, a thin and light hair that after birth replaces the fetal or lanugo hair, and (2) the terminal hair, thicker and pigmented hair that includes the scalp, face, eyelids, trunk, armpits, groin, and extremities [3].
The melanocytes are the cells responsible for the pigmentation of the hair and are embryologically derived from a germinal population of melanoblasts originating from within the neural crest cells, shortly after neural tube closure [4]. The melanoblasts migrate from the neural crest stem, following a dorsolateral path between the dermatome of the somites and the ectoderm, to their destination in the basal layer of the epidermis or the hair follicle [5]. Other melanocytes, which are derived from the neural crest, migrate to areas around the eye and the stria vascularis in the inner ear. A cell subpopulation differs from the neuroectoderm in situ to become the retinal pigment epithelium. In addition, some melanocytes migrate to the leptomeninges and the mucosa (Fig. 12.2).
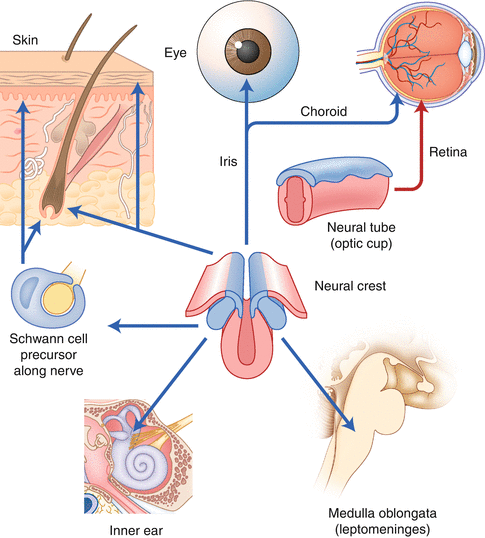

Fig. 12.2
Migration of melanocytes from the neural crest
12.1.2 Anatomy
Schematically, the hair follicle is divided into two segments (Fig. 12.3): the upper segment, which is a permanent part of the follicle and extends from the insertion point of the arrector pili muscle of the follicle up to the skin surface. The upper segment is comprised of two subdivisions: the isthmus, which comes from the insertion point of the arrector pili muscle up to the opening of the sebaceous gland in the hair follicle, and the infundibulum, which extends from the opening of the sebaceous gland to the skin surface. The opening of the infundibulum on the skin surface is called the follicular ostium or acrotrichum [6].
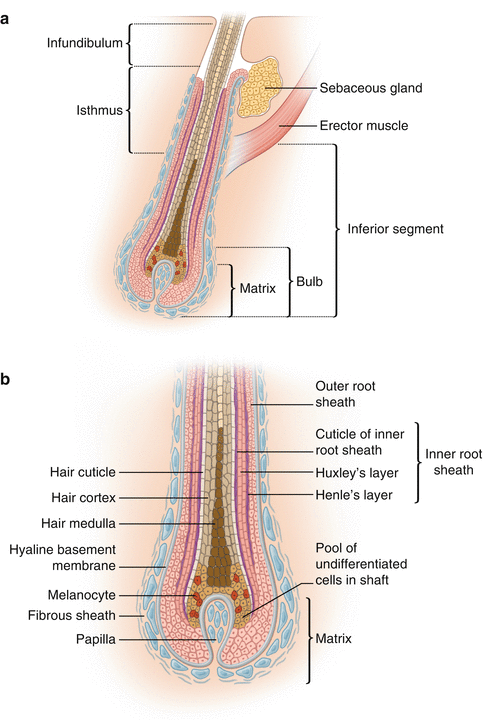

Fig. 12.3
(a) Hair follicle. Longitudinal section showing the three sections: the infundibulum, the isthmus, and the inferior segment. (b) Hair bulb. The outer and inner root sheaths mold and protect the growing hair shaft. The hair shaft consists of the medulla, hair cortex, and cuticle
The part of the hair follicle between the insertion point of the arrector pili muscle to the base of the bulb is called the inferior segment or transient follicle and as is the case in the upper segment is divided into two parts: (1) a stem that extends from the insertion point of the arrector pili muscle to the Adamson’s fringe (the transition region between the “fully cornified” cells, i.e., dead cells, which constitute the hair shaft, and (2) the bulb, which corresponds to the region of Adamson’s fringe, up to the base of the follicle. The bulb base, shaped as a “cup,” contains the matrix and involves the dermal papilla. The dermal papilla is the only part of the lower segment that is not transitory. It is in the lower or transient segment that the basic physiology of the hair occurs, i.e., the biological cycle of the hair follicle [6].
12.2 Growth Cycle
In humans, hair loss occurs in a continuous and asynchronous manner, due to the interaction of numerous growth factors, including cytokines, hormones, neurotransmitters, and their receptors. The hair cycling affects the terminal follicles and the vellus hair, drastically modifying the morphology of the lower segment of the follicle, in addition to connective tissue, blood vessels, nerves, and the cell populations connected with the hair follicle [7]. The hair cycle is divided into three phases (Fig. 12.4):
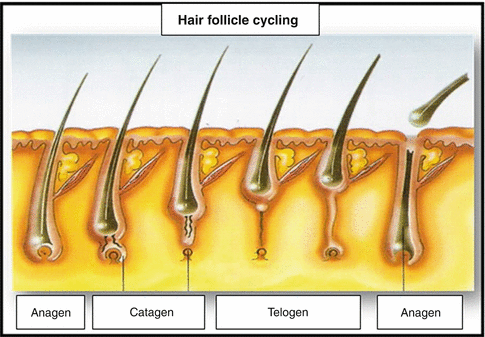

Fig. 12.4
Hair follicle cycling
12.2.1 Growth (Anagen)
It is the longest phase of active growth, enduring from 2 to 7 years, and which may result in hair 12 to 80 cm long. This phase is divided into six phases – I to VI. Its duration varies from one area to another on the body and is the most important determining factor to define the length of the hair. This process may continue for several years in the scalp and only endures a few weeks in the hair follicles of the extremities [1]. The hair bulb of the follicles in the anagen phase presents an abundant production of melanin and an intense mitotic activity that may result in stem growth of about 1 cm per month or 0.35 mm per day. The hairs, which grow more rapidly in humans, are those found in the follicles of the chin, about 0.38 mm/day [8]. About 80–100 % of the hair follicles are in the anagen phase. Due to the fact that intense mitotic activity occurs during this phase, the DNA and melanin synthesis is the phase which is most susceptible to hormonal changes, medications, and toxics in general. It is the only phase during which hair pigmentation occurs as a result of the internalization of bulbar melanocytes, keratinocytes, and fibroblasts of the dermal papilla [1].
12.2.2 Cessation (Catagen)
During this phase, the inferior segment of the follicle regresses sharply due to massive apoptosis of the follicular epithelium, with a remarkable reduction of it in size. This is the shortest phase in the hair cycle and lasts only 2–3 weeks. Consequently, only between 1 and 2 % of follicles develop in this phase and follicles may therefore rarely be encountered in biopsies of the scalp. A horizontal cut of this hair is characterized by oval- or round-shaped hair follicles, abundant apoptosis, and an absence of mitotic activity and melanin pigment production. Forced traction induces the catagen phase. The best time to perform biopsies in order to observe this phenomenon is when biopsies are performed 2–3 months after the occurrence of a traumatic event [1].
12.2.3 Rest (Telogen)
This phase lasts for about 100 days. Between 10 and 20 % of all hair grow during this phase. On the scalp, about 100 hair follicles in the telogen phase are lost per day. Follicles on the chest and extremities have a more frequent and lasting telogen phase than the hair follicles of the scalp. This phase is the final phase of involution of the inferior segment of the hair follicle. Development during this phase consists of two other stages that represent the final components of the telogen phase and that may not be easily detected in routine histological studies:
1.
Exogenous (Teloptose): hair loss without traction occurs spontaneously each day (approximately 100).
2.
Xenogenous: the time interval after the exogenous stage in which the hair follicle remains empty before a new follicle in the anagen phase starts to grow. The frequency and the duration of loss of hair follicles during this phase increase in women and men with androgenetic alopecia [1].
12.3 Physiology of Aging
12.3.1 Changes in Scalp Hair Caused by Aging
According to Accursio [34], the hair aging process includes canities (loss of hair color) and a reduction in the number of hair follicles. Remaining hair is smaller in diameter and grows slower. The resulting baldness is primarily androgenetic [9]. The rate of hair growth varies during human aging.
The onset and progression of hair whitening strongly correlate with chronological age and occurs in various degrees in all individuals, regardless of gender and race. The age of onset is genetically inheritable and controllable. Thus, the age range for Caucasians is, on average, around 30; for Asians after 30; and for Africans, on average, at 40. In fact, premature graying only occurs before the age of 20 in Caucasians, before the age of 25 in Asians, and before the age of 30 in Africans [10].
The darker the hair, the earlier it may turn gray. Scalp hair first turns gray at the temples, spreading to the vertex and subsequently across the head. The occipital region is the last to be affected [11]. The epidermal melanocyte units become less stable and reduce by 10–20 % in its pigment production by the epidermal melanocytes (whether in sun-exposed skin or not) in each decade after the age of 30.
The bulb melanocytes on the scalp engage in greater activity during the years of youth when the follicular melanin unit only experiences aging during a few of its growth cycles [13]. There is empirical evidence to suggest that the capacity of the reservoir of stem cells in the hair follicle may be limited in adults. Repeated removal of the vibrissae of mice possibly caused in an increase of gray hair [14].
Follicular melanocytes differ from epidermal melanocytes by their larger size and longer dendrites and only relate to four to five keratinocytes, as opposed to 36–40 in the case of epidermal melanocytes [15]. Just as the close correlation between keratinocytes and melanocytes in the epidermal melanin units of the skin, the likelihood exists that the hair bulb melanocytes in the follicular melanin unit also influence their neighboring pre-cortical keratinocytes in many ways [12]. For example, the transfer of melanin to the keratinocytes appears to reduce potential of late proliferation and may actually stimulate their differentiation. This suspended proliferation interrupted modulation of keratinocytes, in association with melanin, appears to cause a slight increase in gray and white hair follicles. In fact, white facial hair appears to grow faster than the adjacent hair with color in vivo [16]. Furthermore, white hair follicles display a high degree of elongation of the hair fiber in vitro compared to pigmented hair follicles [17].
12.3.2 Canities
Canities (hair color loss) results from a marked reduction in the melanogenic activity of melanocytes in the hair bulb of hair follicles in the anagen phase. The depigmented follicles emerge on the skin surface already as white hair (Fig. 12.5). “Gray” hair may only be illusory, and in many cases the appearance of gray stems from the mixture of white and pigmented hair. However, gray follicles can also affect individual hair follicles during a short growth stage in anagen phase VI, during which a gradual loss of pigmentation along the stem occurs. In these true gray hair follicles, melanin granules can quickly be detected within the pre-cortex and capillary fiber [11]. These gray follicles display a reduced but detectable dopamine oxidation reaction (an indication of the tyrosinase activity), whereas white hair bulbs appear greatly reduced [18].
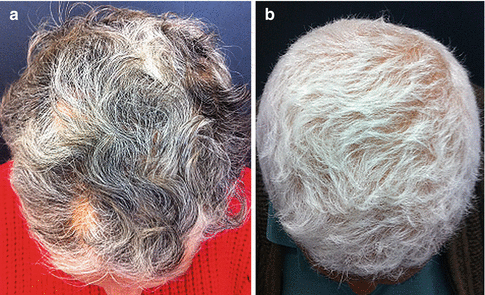

Fig. 12.5
(a) Macroscopic view of graying of the scalp showing admixture of white, gray, and pigmented hair in a woman. (b) Total depigmented hair (canities) in a man (Reprinted with permission from Tobin [11]. © 2008 The Author. Journal compilation. © 2008 Society of Cosmetic Scientists and the Société Française de Cosmétologie)
The extremely low turnover of melanocytes in the epidermis (even after sun exposure) may indicate that the epidermal melanocytes are cells that live a relatively long life. This may be a result of the high concentration of Bcl-2 (antiapoptotic protein) in epidermal melanocytes, which may allow these cells to survive exogenous aggressions, including the influence of reactive oxygen species (ROS) and the endogenous oxidative stress generated during the actual melanogenesis [19]. In instances where this protein is deficient, loss of melanocytes is accelerated through apoptosis when the follicle enters the telogen phase.
There appears to be a loss of dopamine-positive melanocytes with the onset of age across the body surface, not only on the skin (epidermis and hair follicles) but also on the nevi and eyes. However, this pigment reduction is gradual in the epidermis and more dramatic in the hair follicle. Under these conditions, different subpopulations of melanocytes are regulated by different melanogenic clocks. The dominant factor appears to be hereditary and many canities seem to be inherited in an autosomal dominant pattern; this would explain all blood relatives who have gray hair at an early age [20].
The replicative potential loss of melanocytes in vitro is not only associated with increased age of the donor but also with the extensive manipulation of melanin within the cell [21]. The loss of proliferative capacity in hyperpigmented cells appears to result from its inability to activate the mitogen-activated protein (MAP) kinase that is required for proliferation [22]. Upon reaching senescence, melanocytes also express increased levels of inhibitors of cyclin-dependent kinases, which inhibit the cell cycle [23].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree





