I. GENERAL INFORMATION
A. Unlike flaps, grafts do not bring independent blood supply to a recipient bed
1. *Autograft: From same individual
2. *Allograft: From another individual of same species (aka homograft/cadaver graft)
3. *Xenograft: From another species (aka heterograft)
B. Skin, dermis, fat, bone, tendon, cartilage, nerve, fascia, or combinations of tissues can be transferred as grafts
II. ANATOMY
A. Skin: Composed of epidermis and dermis
1. Epidermis: 5% of skin thickness
2. Dermis: 95% of skin thickness, contains sebaceous glands
B. Subcutaneous fat: Deep to dermis, contains hair follicles and sweat glands
III. EVALUATION
A. History: Nutritional status, age, comorbid conditions, smoking status, anticipated compliance
B. Physical exam
1. The recipient site should be assessed for potential bacterial load, blood supply, presence of devitalized tissue, and exposed vital structures.
2. Donor site availability
3. *Perform recipient site tissue culture if history or concern for infection (counts <105 CFU/g tissue for most pathogens required before grafting).
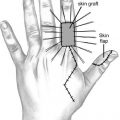
IV. SKIN GRAFTS
A. Indications
1. Primary closure not feasible
2. Lack of adjacent tissue for coverage (poor quality, insufficient quantity, and inferior aesthetic appearance)
3. Uncertain tumor clearance
4. Patients with significant comorbid conditions who may not tolerate the potential risks or complications of more complex reconstructive options.
B. Contraindications
1. Infected recipient bed
2. Unreliable vascularization from recipient bed (e.g., history of radiation)
3. Repeated motion or trauma to recipient bed
4. Exposed white/avascular structures (tendon, nerve, bone, and cartilage) in recipient bed; grafts can technically be placed on paratenon, periosteum, and perichondrium, but typically do not provide durable coverage
5. Anticipated staged reconstruction beneath recipient bed (nerve, tendon reconstruction).
C. Benefits (in comparison with healing by secondary intention): Faster healing, less scar contraction, improved aesthetic appearance, and less fluid loss
______________
*Denotes common in-service examination topics
D. Recipient site requirements
1. Wound site preparation critical to success of graft in order to rid the recipient bed of devitalized tissue and contamination.
a. Viability: Adequate blood supply, no devitalized tissue
b. Hemostasis: Hematoma is the major cause of graft failure
c. Bacterial load: Contamination prevents graft take
2. Patient comorbidities: Systemic diseases/conditions (diabetes, smoking, use of anticoagulants, and nicotine) and local conditions (prior radiation and venous/ arterial insufficiency) can impair graft survival.
E. Donor site considerations: Skin is best replaced with like skin. Example: A skin graft to cover an eyelid defect requiring a thin skin graft is best harvested from “like” tissue, such as preauricular or cervical skin, rather than thicker skin harvested from the inguinal region.
F. General application principles
1. Harvest graft based on the size of defect or slightly larger to account for primary contraction (see below).
2. Graft is secured to skin edges and base of recipient bed with staples, suture (usually chromic or absorbable monofilament), or skin glue.
3. Recipient site dressing and postoperative care
a. Key to bolster is ability to keep the graft in contact with the donor site (tie over or staple into place).
b. Should provide uniform pressure to prevent seroma, hematoma, and shear.
c. Bolster dressing commonly made of nonadherent layer (Xeroform), covered with cotton balls, secured to wound with staples or tie over sutures; a vacuum-assisted closure device can also be used depending on the size and location of the wound, though a nonstick layer (Xeroform (±) Acticoat) should be placed between the vac sponge and graft.
d. Can include additional layer of Acticoat or silver-impregnated gauze if concern for high risk of infection.
e. Bolster not used when graft placed over a transferred muscle flap (e.g., soleus flap for lower extremity defect) due to undesired compression and need to check flap viability.
f. Elevate and immobilize recipient site if possible.
g. Dressing left undisturbed for 4 to 5 days unless shows signs of infection.
h. After bolster removal, BID to QD Xeroform dressing changes ± antibiotic ointment until healing is complete (approximately 2 to 3 weeks) to prevent desiccation.
i. Graft is fragile for several weeks and should be protected from shear forces and edema even after initial bolster is removed.
4. Excess harvested skin may be stored on donor site or at 4°C for several weeks (viability of graft decreases with time) to use for delayed application.
5. May delay graft application for several days with coverage of muscle flaps to facilitate early monitoring of viability.
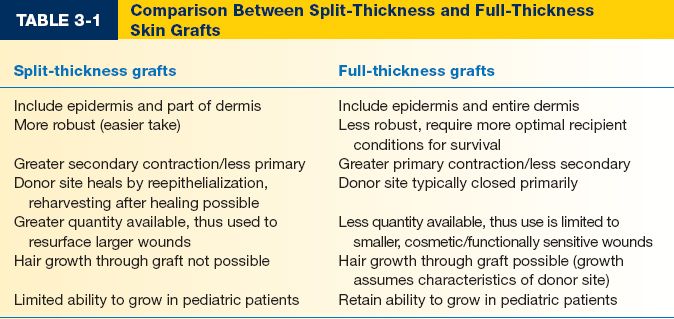
G. Split-thickness skin grafts (STSGs) (Table 3-1)
1. Contain epidermis and variable thickness of dermis; thicker grafts contain more donor skin characteristics but require more optimal recipient site conditions for survival.
2. More robust and available in larger quantity compared with full-thickness skin grafts (FTSGs).
3. Indications: Resurface large wounds, cavities, mucosal defects, muscle flap coverage, flap donor site closure, and temporary closure of wounds after tumor extirpation pending margin clearance with less donor site morbidity compared with other reconstructive options.
4. Donor site
a. Location based on patient preference for location of scar, ease of donor site care, anticipated match of donor skin to recipient site, and availability of sufficient quantity of tissue.
b. Typical sites: Anterolateral thigh, back, abdomen, upper inner arm, and scalp
c. Heals spontaneously by reepithelialization from epidermal appendages in residual dermis.
d. It is possible to reharvest skin at the same site after donor site is healed; back is the best site for reharvesting.
e. Can use tumescence to flatten out area of harvest as well as to decrease blood loss from donor site.
5. Technique
a. Harvest technique options: Free hand knife, drum dermatome, air- or electricity-driven dermatome (most common method).
b. Most grafts are 12/1,000 to 18/1,000 inches thick (infants, elderly, and immunocompromised patients may have thin skin, thus should consider patient and recipient site needs when choosing thickness).
c. Mineral oil is applied to donor site to prepare for harvest
d. Meshed versus sheet grafts
i. Meshed grafts
a) Increase surface area of graft while decreasing harvest area
b) Improve contour of grafts over irregular surfaces
c) Allow for drainage of exudate and blood
d) Increase secondary contraction (may be desired in some locations, but should be avoided over joints and face); 1.5:1 meshing is most commonly used to allow egress of fluid or blood
ii. Sheet (unmeshed) grafts:
a) Provide superior aesthetic benefit
b) Used in face and hands
c) May need pie crusting (small holes) depending on graft size to allow egress of fluid or blood
iii. The larger the meshing, the worse the aesthetic outcome, though interstices will fill in over time
iv. Even if you mesh the graft, it is better if you do not spread it out widely to decrease contraction and to improve aesthetic outcome
6. Donor site care
a. Options: Occlusive dressings (e.g., Duoderm), semiocclusive dressings (e.g., Tegaderm), and semiopen dressings (e.g., Xeroform and Mepilex)
b. *Semiocclusive dressings encourage faster reepithelialization, least painful, nearly maintenance free, and keep wound moist.
c. Semiopen dressings reliable but require daily drying.
d. Watch for infection that can convert a donor site wound from partial- to full-thickness injury.
H. FTSGs (Table 3-1)
1. *Contain epidermis and dermis in entirety and therefore undergo more primary contraction but less secondary contraction than STSGs.
2. Indications: Limited to small, uncontaminated and well-vascularized wounds, generally preferred in cosmetic or functionally sensitive sites (e.g., joints, hands, and face); preferable when color match, thickness, and resistance to contraction are important qualities.
3. Require better vascularized recipient bed due to thickness of graft.
4. Donor site selection
a. Should be inconspicuous and easily closed primarily.
b. Texture, thickness, pigmentation, and presence/absence of hair are important in selection.
c. Common harvest sites: Preauricular, supraclavicular, forehead sites for head and neck recipient sites; groin, lower abdomen, and medial forearm for hand recipient sites.
5. Technique
a. Recipient site preparation is same as with STSG application (see above).
b. Harvest of FTSGs usually done in ellipse shape to facilitate primary closure of donor site.
c. Aggressive defatting of donor skin critical to improve initial survival.
d. Recipient site: Bolster dressing applied as in STSG (see above)
6. Tissue expansion of lower abdomen or groin prior to FTSGs can be used to allow for primary closure of larger graft harvest.
I. Skin graft survival and healing
1. *Imbibition (first 24 to 48 hours): Plasma imbibition (diffusion) responsible for skin graft survival until angiogenesis occurs → thinner grafts more likely to survive.
2. *Inosculation (48 to 72 hours): Process of capillaries joining between skin graft and recipient bed.
3. Revascularization (4 to 7 days): Ingrowth of capillaries into graft
4. Primary contraction
a. Occurs at the time of graft harvest/application
b. Due to elastin fibers in dermis
c. Greater in FTSGs (>40%) compared with STSGs (<20%)
5. Secondary contraction
a. Occurs after graft take
b. During healing phase of graft over 6 to 18 months
c. Greater in STSGs
d. Dermal components of FTSGs suppress myofibroblast activities responsible for secondary contraction
6. Regeneration of dermal appendages
a. More likely to regenerate in thicker grafts
b. Sweating assumes characteristics of recipient site when glands are reinnervated
c. Sebaceous glands retain characteristics of donor site
7. Reinnervation
a. Begins 2 to 4 weeks after grafting
b. Process takes several months to years
c. Assumes characteristics of recipient site
d. Reinnervation incomplete and some degree of decreased sensation will persist
e. STSGs regain sensation quicker, but FTSGs regain more complete innervation.
f. Pain returns first, then touch, then temperature.
8. Hair growth: Assumes characteristics of donor site, but only has potential to return after FTSGs.
9. Pigmentation
a. More predictable in FTSGs
b. Permanent hyperpigmentation may result from early sun exposure before full maturation.
10. Growth potential: STSGs have limited ability to grow in pediatric patients, FTSGs have potential to grow.
J. Skin graft complications
1. Graft failure (due to hematoma, seroma, infection, poor graft fixation, and smoking)
2. Pigment changes (donor and recipient sites), scar contraction, hypertrophic scarring, and graft instability
V. BONE GRAFTS
A. Classifications
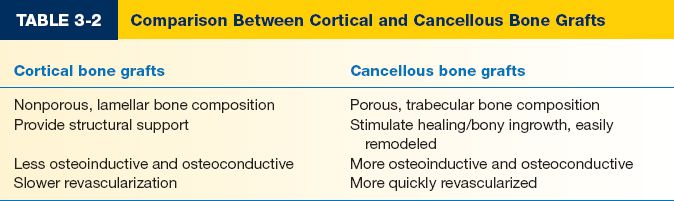
1. By composition (Table 3-2)
a. Cortical: Composed of nonporous; lamellar bone; primarily used for support of major bony defects; more osteogenesis.
b. *Cancellous
i. Composed of porous, trabecular bone
ii. Used to stimulate healing/bony ingrowth
iii. Bridge smaller defects, increase bulk
iv. Offers little structural support, easily remodeled
v. More osteoinductive and osteoconductive (see below) and more quickly revascularized than cortical bone graft
c. Corticocancellous: Theoretically provides benefits of both
2. By vascular supply
a. Nonvascularized: Provides scaffolding and template for vascular and cellular ingrowth; ingrowth eventually resorbs and replaces graft (creeping substitution or osteoconduction).
b. Pedicled vascularized: Bone graft transferred on vascular pedicle.
c. Free vascularized: Transfer of large segment of bone, promotes healing at recipient site, retains epiphyseal growth.
3. By origin
a. Autograft: Maximal healing potential, increased surgical time, no risk of viral transmission
b. Allograft
i. From cadaveric sources
ii. Readily available, avoids donor site morbidity, increased time for incorporation due to immunogenicity
iii. Freeze-dried allografts have higher availability, lower immunogenicity, and lower risk of disease transfer compared with fresh-frozen allografts
c. Bone graft substitutes: All have minimal structural integrity (e.g., bone morphogenic protein [BMP])
B. Indications
1. Promote and enhance healing: Delayed union, nonunion, osteotomies, or other sites of poor healing potential
2. Bridge bony defects: Fill cortical defects (comminuted fractures and tumor excision), provide continuity
3. Fill cavities: In cases of cyst, tumor, or sequestrum removal
4. Arthrodesis: Replacement of native joint with bone graft
5. Provide structural support to implanted devices
C. Donor sites: Selection depends on quantity, type, vascularity of bone desired, donor site morbidity, and patient characteristics
1. Ilium: Large quantity cancellous and corticocancellous bone; inner or both tables of iliac crest available for harvest with additional cancellous bone available by curettage; vascularized graft based on deep circumflex iliac artery can be used
a. Use osteotome to lift cartilage cap superior to anterior, superior iliac spine coming from lateral aspect of iliac rim.
b. Advantages: Little aesthetic deficit, limited use of cortical bone in patients <10 years old due to incomplete ossification.
c. Disadvantage: Donor site pain
2. Cranium (along the origin of temporalis muscle if possible where calvarium thickest): Large quantity of cortical bone (outer table used in adults; both inner and outer tables used in children due to osteogenic potential of dura)
a. Advantages: Low graft resorption, low donor morbidity, and good aesthetic result.
b. Disadvantages: Brittleness, larger bone grafts require formal craniotomy.
3. Ribs (11th and 12th): Cortical bone that is more porous and malleable than graft from other sources.
a. Advantages: Malleable, can split in half
b. Disadvantages: Difficult fixation due to porosity
4. Fibula: Pedicled or free graft based on peroneal artery and venae comitantes; bridges defects in long bones. Important to leave cuff of fibula proximal proximally and distally.
a. Advantages: Good graft length, long pedicle, and little functional deficit
b. Disadvantages: Limited size
5. Other sites: Distal radius, proximal ulna for cortical and cancellous bone
D. Harvesting and recipient site preparation tips
1. Minimize time between harvest and placement
2. Graft should be kept wrapped in blood-soaked sponges
3. Use copious irrigation during sawing and drilling to reduce mechanical and thermal damage to bone.
4. Bone edges at recipient site should be freshened to bleeding edges to ensure potential for revascularization of graft.
E. Graft survival and healing
1. *Osteoconduction: Scaffold or template function that graft provides to allow ingrowth of capillaries, osteoprogenitor cells, and matrix components from host tissue. (Example: Non vascularized bone graft)
2. *Osteoinduction: Growth factors (BMPs) present within graft recruit host stem cells to form bone-producing cells (osteoblasts). (Example: Cancellous bone)
3. *Osteogenesis: Production of new bone by cells in graft that survive transplantation. (Example: Vascularized bone graft)
4. Costochondral bone grafts retain ability to grow and may grow excessively.
VI. CARTILAGE GRAFTS
A. Classifications
1. By matrix characteristics
a. Hyaline cartilage: Trachea, larynx, nasal septum, nasal ala, and ribs; offers support through rigidity
b. Elastic cartilage: External ear, external auditory meatus, eustachian tube, and epiglottis; more malleable, elastic, more resistant to repeated bending than hyaline cartilage
c. Fibrocartilage: Pubic symphysis, intervertebral disks, ligamentous and tendinous insertions; resists tensile and compressive forces; lacks flexibility
2. By source
a. Autogenous: Primary and preferred source
b. Homologous (cadaveric): Produces relatively small immune response (chondrocytes surrounded by nonreactive extracellular matrix); freeze-dried/preserved cartilage reduces further inflammation/disease transfer; more absorption compared with autogenous cartilage
B. Indications
1. Structural support and augmentation: Ear reconstruction, eyelid and tracheal support
2. Contour deformity: Correction of nasal deformity (e.g., saddle nose) and inverted nipples, alternative to bone graft in facial contour deformities
3. Joint repair and resurfacing: Spacer in temporomandibular joint (TMJ) repair, fill defects in articular cartilage
1. Ear (concha): Elastic cartilage source, possesses natural curvature, used for eyelid support and nipple reconstruction, TMJ, and orbital floor repair
a. Advantages: Easily accessible, abundant
b. Disadvantages: Curvature not always desirable
2. Nasal septum: Straight, rigid, hyaline cartilage source, used for nasal or lower eyelid reconstruction
a. Advantages: Easily accessible
b. Disadvantages: Limited availability, overresection results in saddle-nose deformity
3. Costal cartilage: Abundant source of hyaline cartilage, used for reconstructions requiring large amount of cartilage (total auricular reconstruction, tracheal reconstruction)
a. Advantages: Large quantity graft material, reliable, and distant recipient site allows two-team harvest approach
b. Disadvantages: Tend to warp with time, donor site morbidity (pneumothorax and pain)
D. Graft survival and healing
1. Chondrocytes and extracellular matrix survive and maintain cartilage characteristics
2. Survives by osmosis from well-vascularized recipient site (avascular)
3. Limited inflammatory reaction with little graft resorption (<20% in autografts)
4. Requires coverage to prevent desiccation and infection
5. Scoring allows graft to be shaped (bending away from scored side)
6. Symmetric carving, K-wire stabilization, harvest without perichondrium, making central rather than peripheral cuts, and waiting at least 30 minutes after carving before placement at recipient site can be employed to decrease warping
VII. FAT GRAFTS (SEE CHAPTER 9)
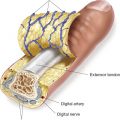
VIII. COMPOSITE GRAFTS
A. Composed of two or more tissue components (e.g., skin or mucosa with cartilage, skin with fat, and full-thickness eyelid)
B. Indications
1. Nasal ala: Prevent alar collapse
2. Nasal sidewall: Prevent nasal valve obstruction
3. Nasal tip: Provide structural integrity
4. Ear (anterior helical root): Repair substantial auricular defects, restoration of ear structure for glasses or hearing aid placement
5. Eyelid: Prevent ectropion and lid contraction from loss of tarsal plate
C. Donor sites: Septal cartilage, auricular cartilage, and costal cartilage
D. Graft survival and healing
1. Survival occurs via imbibition, inosculation, then revascularization
2. Initial survival dependent on revascularization solely from wound edges; thus no portion of the graft should be >1 cm from wound edges
3. Metabolic demand of graft limits size that will survive to 1.0 to 1.5 cm width
4. More prone to graft loss than other graft types
PEARLS
1. Dermal side of skin graft can be distinguished from epidermal surface by its shiny appearance (graft placed shiny side [dermis] down)
2. Donor scars for harvesting of FTSGs should be oriented parallel to relaxed skin tension lines (RSTLs)
3. Harvest of FTSGs from volar wrist should never be performed due to social stigma of wrist scar
4. Patients should be warned that composite grafts often initially appear cyanotic
QUESTIONS YOU WILL BE ASKED
1. Name the stages and timing of skin graft healing?
Imbibition (first 24 to 48 hours), inosculation (48 to 72 hours), and revascularization (4 to 7 days).
2. After skin grafting, does the donor or recipient site determine characteristics of hair growth, sweating, and sensibility?
Hair growth assumes characteristics of the donor site, but only has potential to return after FTSG, sweating assumes characteristics of recipient site when glands are reinnervated, and sensibility is incomplete and assumes characteristics of recipient site.
3. What is the difference between primary and secondary contraction and which type of skin graft is primarily affected by each?
Primary contraction occurs immediately at the time of graft harvest/application due to elastic fibers in dermis, greater in FTSGs; secondary contraction occurs during healing phase of graft over 6 to 18 months, greater in STSGs.
4. Draw the layers of skin from epidermis to subcutaneous fat.
See Figure 1-1
Recommended Readings
Coleman SR. Facial augmentation with structural fat grafting. Clin Plast Surg. 2006;33(4):567–577. PMID: 17085224.
Hallock GG, Morris SF. Skin grafts and local flaps. Plast Reconstr Surg. 2011;127(1):5e-22e. PMID: 21200192.
< div class='tao-gold-member'>