Chapter 56 Graft-Tunnel Healing
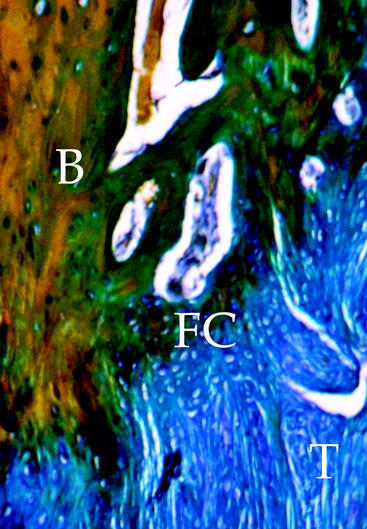
Tendon graft healing within the bone tunnel is one of the most important factors affecting “ligamentization” of the anterior cruciate ligament (ACL) graft, as it contributes to determine the mechanical behavior of the femur–ACL graft–tibia complex. The normal ACL attaches to the bone through a direct-type insertion, which has a highly differentiated morphology. In fact, within 1 mm, four different layers can be recognized: fibrous tissue, fibrocartilage, mineralized fibrocartilage, and bone (Fig. 56-1). This region plays an important mechanical role, as it allows a progressive distribution of the tensile load from a soft tissue (ligament) to a hard tissue (bone).
Human Studies
Despite the large amount of animal studies on bone–tendon graft healing in ACL reconstruction, very few investigations in humans have been reported on this issue. Pinczewski et al1 reported on two biopsies at the bone–graft interface on patients who underwent revision ACL surgery for a traumatic graft failure at 6 and 10 weeks after initial reconstruction with doubled hamstring tendon (HT) graft and fixation with metal interference screws. They described graft integration by way of collagen fibers resembling Sharpey fibers between tendon and bone. Petersen and Laprell2 compared bone–tendon graft healing after ACL reconstruction between the patellar tendon (PT) and HT on biopsy specimens obtained at ACL revision surgery from 14 patients. They observed that the PT graft healed within bone tunnel by bone plug incorporation, maintaining a direct-type insertion at the native bone plug–tendon junction. Tendon–bone healing occurred by formation of a fibrous insertion with no evidence of fibrocartilage. Ishibashi et al3 examined the histological changes in PT autografts at the tibial tunnel in biopsy specimens retrieved during revision surgery after ACL reconstruction in 10 patients. They observed that in the early revisions (less than 1 year from prior reconstruction), the bone–tendon junction was still immature, with presence of granulation tissue between the tendon and the tunnel wall; in the late revisions (more than 1 year), the original bone–tendon junction was not seen, and the tendon continued completely to the tunnel wall with Sharpey-like fibers. Nebelung et al4 obtained biopsies from the femoral tunnel in five patients at 6 to 14 months after ACL reconstruction with HT. Fixation was performed in four patients with a suspension device (Endobutton or TransFix) and in one patient with an interference screw. At histology of the four reconstructions with a suspension device, biopsies resembled granulation tissue without continuity of collagen fibers between the graft and the bony wall. In contrast, in the graft fixed with interference screws, a metaplastic fibrous cartilage between the tendon graft and the lamellar bone was noted. The authors hypothesized that suspensory fixation can produce micromotion at the tendon–bone interface, which can impair graft healing within the bone tunnel. Robert et al5 performed 12 biopsies on patients undergoing an arthroscopy between 3 and 20 months after ACL reconstruction with HT and femoral fixation with a suspension device (TransFix). Histological analysis at 3 months showed a fibrovascular interface and an uncalcified osteoid with very few collagen fibers between the tendon and the bone. At 5 and 6 months, some Sharpey-like fibers and less immature woven bone were seen. Maturity of insertion with numerous Sharpey fibers at the tendon–bone interface was seen by 10 months. After 1 year, the tendon–bone interface was composed of a continuous layer of Sharpey-like fibers. In three cases, no contact was seen at biopsy despite good clinical stability at 1 year. The authors concluded that suspensory femoral fixation of HT graft produces an indirect fixation that reaches maturity 10 to 12 months after reconstruction.
Animal Studies
Third, studies on tendon–bone healing differ in the methods of investigation of the results. Several authors performed a histological examination of the bone–tendon graft interface,6–13 whereas others focused on the mechanical properties of the bone–tendon graft complex.14–19 It has to be considered that most of the biomechanical studies were based on a load-to-failure (LTF) testing of the femur–graft–tibia complex and did not consider the effect of primary fixation on the structural properties of the complex. For this reason, some authors have performed mechanical testing after removal of the fixation devices in order to quantify the mechanical role of bone–tendon graft interface.14,15,17–19 Moreover, almost all the experimental studies performed histological and/or biomechanical evaluations at different time intervals, and although some authors evaluated the long-term fate of tendon graft healing within a bone tunnel,8,10,11,13,16,20 most authors focused on the first 12 weeks after surgery because of the clinical relevance of this period for planning postoperative rehabilitation and return to physical activity.
Type of Graft
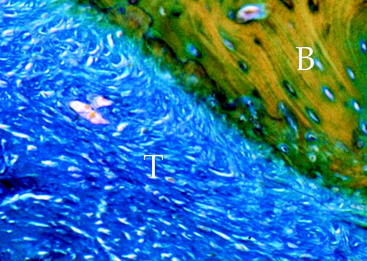
Several experiment studies focused their attention on histological and biomechanical findings of bone–tendon graft healing according to the type of tendon graft. The first reports on tendon graft healing within a bone tunnel showed that it happens through bone apposition at the tunnel wall and formation of fibrous tissue at the bone–graft interface, which matures with time and anchors the graft to the bone.21–23 More recent studies on extraarticular animal models6,9 demonstrated that tendon graft heals within a bone tunnel by formation of an indirect-type junction composed of a fibrous tissue containing perpendicular collagen fibers resembling Sharpey-like fibers that penetrate into the bone, without a transitional fibrocartilaginous layer between tendon and bone (Fig. 56-2). In a biomechanical analysis, Rodeo et al6 showed that until 8 weeks after surgery, the healing tissue at the bone–graft interface was not mechanically competent and the graft failed due to pullout from the tunnel.
Experimental studies performed on intraarticular models of ACL reconstruction with single or doubled semitendinosus tendon graft confirmed that even an autologous ACL graft heals within the bone tunnel by formation of an indirect-type junction at the bone–graft interface, with Sharpey-like fibers perpendicular to the tunnel wall.7,8,11 The newly formed insertion was evident 8 weeks after surgery and completed after 24 weeks. However, biomechanical testing showed that the graft remains weak within 1 year after surgery, with a mean failure load ranging from 25% to 50% of the normal ACL. Regarding the tensile strength of the bone–tendon graft junction, Grana et al,7 using a single-strand semitendinosus tendon graft in a rabbit model, observed that during the first 3 weeks after surgery the graft suffered a rapid and dramatic loss of its mechanical properties and failed mostly at its midsubstance rather than due to pullout from the tunnel. On the contrary, Goradia et al,11 using a doubled semitendinosus tendon graft in a sheep model, observed that up to 12 weeks, graft failure occurred by pullout from the bone tunnel. Therefore they stated that for as long as 3 months after surgery, the graft has not completely healed within the bone tunnel.
Other authors10,12,15 focused their attention on the healing process of grafts with bone plugs, such as the patellar tendon, within a bone tunnel (bone–bone healing). They observed that graft healing occurs differently for the bone plug and the intraosseous tendinous portion of the graft. In fact, bone plug incorporation at the tunnel wall occurs through a progression of necrosis, resorption, and remodeling, and after 3 months it is no longer distinguishable from the surrounding bone. The intraosseous tendinous portion of the graft heals to the bone tunnel by formation of an indirect-type insertion with penetrating collagen fibers that appear well organized by 3 months after surgery, similarly to the healing process observed for free tendon grafts. The native bone–tendon junction of the graft shows degeneration of the fibrocartilaginous layer by 6 weeks, which is during the phase of bone plug remodeling. However, by 6 months it appears to be redifferentiated with four distinct zones.
Comparative studies between tendon–bone and bone–bone healing on intraarticular models of ACL reconstruction confirmed similar histological findings.14,15,24 However, biomechanical testing demonstrated that bone–bone healing occurs more rapidly than tendon–bone healing. In fact, up to 3 weeks, both soft tissue and bone plug tendon grafts fail due to pullout from the bone tunnel. Between 6 and 8 weeks after surgery, the bone–bone interface appears mechanically stronger than the tendon–bone interface, but this difference is no more significant by 12 weeks. These observations led the authors to conclude that soft tissue grafts such as hamstring tendons heal more slowly than PT within the bone tunnel after ACL reconstruction; therefore the fixation device for soft tissue tendon grafts is more important than comparing it to PT graft during the first weeks after surgery.
Regarding the tendon allograft, experimental studies showed that the bone–graft healing process is similar to that observed for autografts.20,25,26 However, it occurs more slowly and the newly formed bone–tendon junction is evident only after a period varying from 18 weeks to 6 months after surgery. This delayed healing process should be related to the inflammatory response to the allogenic material, which persists for a long time around the graft20 and probably leads to the tunnel-widening phenomenon that mainly occurs during the first weeks after surgery.26
Bone Quality
Another variable that can influence tendon graft healing is the quality of bone where the graft is fixed. It is well known that bone density is different between the distal femur and the proximal tibia; this could affect the quality and rate of incorporation of tendon graft at the cancellous bone surface of the tunnel wall. Some authors11,27 investigated this feature of bone–tendon graft healing on experimental intraarticular and extraarticular models; however, the role of bone quality on bone–tendon graft healing remains unclear. Goradia et al11 performed an ACL reconstruction with doubled semitendinosus tendon graft and did not observe histological differences in tendon–bone healing between the femoral and tibial tunnel at each interval (from 2 to 52 weeks). On the contrary, Grassman et al,27 using the semitendinosus tendon graft for extraarticular reconstruction of the medial collateral ligament in a rabbit model, observed that incorporation and remodeling of the graft within the bone tunnels were much more extensive at the cancellous-filled femoral bone–graft interface than within the marrow-dominated tibial tunnel, thus suggesting that tendon graft healing may depend on the cancellous bone architecture at the bone–graft interface.
Fixation Technique
Most studies performed to evaluate tendon–bone healing did not consider graft fixation technique as a factor affecting the healing process. Particularly, many authors reported the use of periosteal or transosseous sutures for graft fixation.6–928 This fixation technique cannot guarantee high structural properties of the bone–tendon graft complex before biological fixation has occurred. Recently, some authors investigated the role of primary fixation on bone–tendon healing, reproducing in animal models some fixation techniques currently used in humans for ACL tendon grafts. Weiler et al13,16 performed a histological and biomechanical evaluation of healing of a tendon graft fixed within the tibial tunnel with an interference fit screw (1 mm larger than the tunnel) after an ACL reconstruction with autologous Achilles tendon split graft in a sheep model. They observed that bone–tendon healing, under the compressive effect of the interference screw, progressed partially by direct contact without development of a fibrous transition interface, whereas at the articular tunnel aperture site a well-differentiated, direct-type junction was evident by 24 weeks.13 Biomechanical testing16 showed that at 6 and 9 weeks, all grafts failed at the screw insertion site. By 24 weeks, grafts failed by osteocartilaginous avulsion from the tibial attachment site. These findings indicate that interference fit fixation may compromise the mechanical properties of the graft in the early healing phase at the screw insertion site, but the compressive effect of the screw supplies a biological stimulus toward the formation of a physiological, direct–type bone–graft insertion. Singhatat et al17
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree