Chapter 1
General Principles
- Embryology, structure and function of the skin
- Blood supply to the skin
- Classification of flaps
- Geometry of local flaps
- Wound healing and skin grafts
- Bone healing and bone grafts
- Cartilage healing and cartilage grafts
- Nerve healing and nerve grafts
- Tendon healing
- Transplantation
- Tissue engineering
- Alloplastic implantation
- Wound dressings
- Sutures and suturing
- Tissue expansion
- Lasers
- Local anaesthesia
- Microsurgery
- Haemostasis and thrombosis
- Further reading
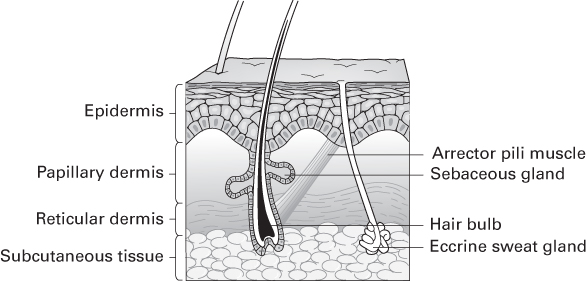
Embryology, structure and function of the skin
- Skin differentiates from ectoderm and mesoderm during the 4th week.
- Skin gives rise to:
- Teeth and hair follicles, derived from epidermis and dermis
- Fingernails and toenails, derived from epidermis only.
- Hair follicles, sebaceous glands, sweat glands, apocrine glands and mammary glands are ‘epidermal appendages’ because they develop as ingrowths of epidermis into dermis.
- Functions of skin:
- Physical protection
- Protection against UV light
- Protection against microbiological invasion
- Prevention of fluid loss
- Regulation of body temperature
- Sensation
- Immunological surveillance.
- Sensation
- Skin gives rise to:
The epidermis
- Composed of stratified squamous epithelium.
- Derived from ectoderm.
- Epidermal cells undergo keratinisation—their cytoplasm is replaced with keratin as the cell dies and becomes more superficial.
- Rete ridges are epidermal thickenings that extend downward between dermal papillae.
- Epidermis is composed of these five layers, from deep to superficial:
- Stratum germinativum
- Also known as the basal layer.
- Cells within this layer have cytoplasmic projections (hemidesmosomes), which firmly link them to the underlying basal lamina.
- The only actively proliferating layer of skin.
- Stratum germinativum also contains melanocytes.
- Also known as the basal layer.
- Stratum spinosum
- Also known as the prickle cell layer.
- Contains large keratinocytes, which synthesise cytokeratin.
- Cytokeratin accumulates in aggregates called tonofibrils.
- Bundles of tonofibrils converge into numerous desmosomes (prickles), forming strong intercellular contacts.
- Also known as the prickle cell layer.
- Stratum granulosum
- Contains mature keratinocytes, with cytoplasmic granules of keratohyalin.
- The predominant site of protein synthesis.
- Combination of cytokeratin tonofibrils with keratohyalin produces keratin.
- Contains mature keratinocytes, with cytoplasmic granules of keratohyalin.
- Stratum lucidum
- A clear layer, only present in the thick glabrous skin of palms and feet.
- Stratum corneum
- Contains non-viable keratinised cells, having lost their nuclei and cytoplasm.
- Protects against trauma.
- Insulates against fluid loss.
- Protects against bacterial invasion and mechanical stress.
- Contains non-viable keratinised cells, having lost their nuclei and cytoplasm.
Cellular composition of the epidermis
- Keratinocytes—the predominant cell type in the epidermis.
- Langerhans cells—antigen-presenting cells (APCs) of the immune system.
- Merkel cells—mechanoreceptors of neural crest origin.
- Melanocytes—neural crest derivatives:
- Usually located in the stratum germinativum.
- Produce melanin packaged in melanosomes, which is delivered along dendrites to surrounding keratinocytes.
- Melanosomes form a cap over the nucleus of keratinocytes, protecting DNA from UV light.
- Usually located in the stratum germinativum.
The dermis
- Accounts for 95% of the skin’s thickness.
- Derived from mesoderm.
- Papillary dermis is superficial; contains more cells and finer collagen fibres.
- Reticular dermis is deeper; contains fewer cells and coarser collagen fibres.
- It sustains and supports the epidermis.
- Dermis is composed of:
- Collagen fibres
- Produced by fibroblasts.
- Through cross-linking, are responsible for much of the skin’s strength.
- The normal ratio of type 1 to type 3 collagen is 5:1.
- Produced by fibroblasts.
- Elastin fibres
- Secreted by fibroblasts.
- Responsible for elastic recoil of skin.
- Secreted by fibroblasts.
- Ground substance
- Consists of glycosaminoglycans (GAGs): hyaluronic acid, dermatan sulphate, chondroitin sulphate.
- GAGs are secreted by fibroblasts and become ground substance when hydrated.
- Consists of glycosaminoglycans (GAGs): hyaluronic acid, dermatan sulphate, chondroitin sulphate.
- Vascular plexus
- Separates the denser reticular dermis from the overlying papillary dermis.
Skin appendages
Hair follicles
- Each hair is composed of a medulla, a cortex and an outer cuticle.
- Hair follicles consist of an inner root sheath (derived from epidermis), and an outer root sheath (derived from dermis).
- Several sebaceous glands drain into each follicle.
- Drainage of the glands is aided by contraction of arrector pili muscles.
- Vellus hairs are fine and downy; terminal hairs are coarse.
- Hairs are either in anagen (growth), catagen (regressing), or telogen (resting) phase.
- <90% are in anagen, 1–2% in catagen and 10–14% in telogen at any one time.
Eccrine glands
- These sweat glands secrete odourless hypotonic fluid.
- Present in almost all sites of the body.
- Occur more frequently in the palm, sole and axilla.
Apocrine glands
- Located in axilla and groin.
- Emit a thicker secretion than eccrine glands.
- Responsible for body odour; do not function before puberty.
- Modified apocrine glands are found in the external ear (ceruminous glands) and eyelid (Moll glands).
- The mammary gland is a modified apocrine gland specialised for manufacture of colostrum and milk.
- Hidradenitis suppurativa is a disease of apocrine glands.
Sebaceous glands
- Holocrine glands that drain into the pilosebaceous unit in hair-bearing skin.
- They drain directly onto skin in the labia minora, penis and tarsus (meibomian glands).
- Most prevalent on forehead, nose and cheek; absent from palms and soles.
- Produce sebum, which contains fats and their breakdown products, wax esters and debris of dead fat-producing cells.
- Sebum is bactericidal to staphylococci and streptococci.
- Sebaceous glands are not the sole cause of so-called sebaceous cysts.
- These cysts are in fact of epidermal origin and contain all substances secreted by skin (predominantly keratin).
- Some maintain they should therefore be called epidermoid cysts.
Types of secretion from glands
- Eccrine or merocrine glands secrete opened vesicles via exocytosis.
- Apocrine glands secrete by ‘membrane budding’—pinching off part of the cytoplasm in vesicles bound by the cell’s own plasma membrane.
- Holocrine gland secretions are produced within the cell, followed by rupture of the cell’s plasma membrane.
Histological terms
- Acanthosis: epidermal hyperplasia.
- Papillomatosis: increased depth of corrugations at the dermoepidermal junction.
- Hyperkeratosis: increased thickness of the keratin layer.
- Parakeratosis: presence of nucleated cells at the skin surface.
- Pagetoid: when cells invade the upper epidermis from below.
- Palisading: when cells are oriented perpendicular to a surface.
- Pagetoid: when cells invade the upper epidermis from below.
Blood supply to the skin
- Epidermis contains no blood vessels.
- It is dependent on dermis for nutrients, supplied by diffusion.
Anatomy of the circulation
- Blood reaching the skin originates from named deep vessels.
- These feed interconnecting vessels, which supply the vascular plexuses of fascia, subcutaneous tissue and skin.
Deep vessels
- Arise from the aorta and divide to form the main arterial supply to head, neck, trunk and limbs.
Interconnecting vessels
- The interconnecting system is composed of:
- Fasciocutaneous (or septocutaneous) vessels
- Reach the skin directly by traversing fascial septa.
- Provide the main arterial supply to skin in the limbs.
- Reach the skin directly by traversing fascial septa.
- Fasciocutaneous (or septocutaneous) vessels
- Musculocutaneous vessels
- Reach the skin indirectly via muscular branches from the deep system.
- These branches enter muscle bellies and divide into multiple perforating branches, which travel up to the skin.
- Provide the main arterial supply to skin of the torso.
- Reach the skin indirectly via muscular branches from the deep system.
Vascular plexuses of fascia, subcutaneous tissue and skin
- Subfascial plexus
- Small plexus lying on the undersurface of deep fascia.
- Prefascial plexus
- Larger plexus superficial to deep fascia; prominent on the limbs.
- Predominantly supplied by fasciocutaneous vessels.
- Larger plexus superficial to deep fascia; prominent on the limbs.
- Subcutaneous plexus
- At the level of superficial fascia.
- Mainly supplied by musculocutaneous vessels.
- Predominant on the torso.
- At the level of superficial fascia.
- Subdermal plexus
- Receives blood from the underlying plexuses.
- The main plexus supplying blood to skin.
- Accounts for dermal bleeding observed in incised skin.
- Receives blood from the underlying plexuses.
- Dermal plexus
- Mainly composed of arterioles.
- Plays an important role in thermoregulation.
- Subepidermal plexus
- Contains small vessels without muscle in their walls.
- Predominantly nutritive and thermoregulatory function.
- Mainly composed of arterioles.
Angiosomes
- An angiosome is a three-dimensional composite block of tissue supplied by a named artery.
- The area of skin supplied by an artery was first studied by Manchot in 1889.
- His work was expanded by Salmon in the 1930s, and more recently by Taylor and Palmer.
- The anatomical territory of an artery is the area into which the vessel ramifies before anastomosing with adjacent vessels.
- The dynamic territory of an artery is the area into which staining extends after intravascular infusion of fluorescein.
- The potential territory of an artery is the area that can be included in a flap if it is delayed.
- Vessels that pass between anatomical territories are called choke vessels.
- The transverse rectus abdominis myocutaneous (TRAM) flap illustrates the angiosome concept well:
Zone 1
- Receives musculocutaneous perforators from the deep inferior epigastric artery (DIEA) and is therefore in its anatomical territory.
Zones 2 and 3
- There is controversy as to which of the following zones is 2 and which is 3.
- Hartrampf’s 1982 description has zone 2 across the midline and zone 3 lateral to zone 1.
- Holm’s 2006 study shows the opposite to be true.
- Skin lateral to zone 1 is in the anatomical territory of the superficial circumflex iliac artery (SCIA).
- Blood has to travel through a set of choke vessels to reach it from the ipsilateral DIEA.
- Skin on the contralateral side of the linea alba is in the anatomical area of the ipsilateral DIEA.
- It is also within the dynamic territory of the contralateral DIEA.
- This allows a TRAM flap to be reliably perfused based on either DIEA.
- It is also within the dynamic territory of the contralateral DIEA.
Zone 4
- This lies furthest from the pedicle and is in the anatomical territory of the contralateral SCIA.
- Blood passing from the pedicle to zone 4 has to cross two sets of choke vessels.
- This portion of the TRAM flap has the worst blood supply and is often discarded.
Arterial characteristics
- Taylor made the following observations from his detailed anatomical dissections:
- Vessels usually travel with nerves.
- Vessels obey the law of equilibrium—if one is small, its neighbour will tend to be large.
- Vessels travel from fixed to mobile tissue.
- Vessels have a fixed destination but varied origin.
- Vessel size and orientation is a product of growth.
- Vessels have a fixed destination but varied origin.
- Vessels usually travel with nerves.
Venous characteristics
- Venous networks consist of linked valvular and avalvular channels that allow equilibrium of flow and pressure.
- Directional veins are valved; typically found in subcutaneous tissues of limbs or as a stellate pattern of collecting veins.
- Oscillating avalvular veins allow free flow between valved channels of adjacent venous territories.
- They mirror and accompany choke arteries.
- They define the perimeter of venous territories in the same way choke arteries define arterial territories.
- They mirror and accompany choke arteries.
- Superficial veins follow nerves; perforating veins follow perforating arteries.
The microcirculation
- Terminal arterioles are found in reticular dermis.
- They terminate as they enter the capillary network.
- The precapillary sphincter is the last part of the arterial tree containing muscle within its wall.
- It is under neural control and regulates blood flow into the capillary network.
- The skin’s blood supply far exceeds its nutritive requirements.
- It bypasses capillary beds via arteriovenous anastomoses (AVAs) and has a primarily thermoregulatory function.
- AVAs connect arterioles to efferent veins.
- AVAs are of two types:
- Indirect AVAs—convoluted structures known as glomera (sing. glomus)
- Densely innervated by autonomic nerves.
- Direct AVAs—less convoluted with sparser autonomic supply.
- Indirect AVAs—convoluted structures known as glomera (sing. glomus)
Control of blood flow
- The muscular tone of vessels is controlled by:
Pressure of the blood within vessels (myogenic theory)
- Originally described by Bayliss, states that:
- Increased intraluminal pressure results in constriction of vessels.
- Decreased intraluminal pressure results in their dilatation.
- Increased intraluminal pressure results in constriction of vessels.
- Helps keep blood flow constant; accounts for hyperaemia on release of a tourniquet.
Neural innervation
- Arterioles, AVAs and precapillary sphincters are sympathetically innervated.
- Increased arteriolar tone results in decreased cutaneous blood flow.
- Increased precapillary sphincter tone reduces blood flow into capillary networks.
- Decreased AVA tone increases non-nutritive blood flow bypassing the capillary bed.
Humoral factors
- Epinephrine, norepinephrine, serotonin, thromboxane A2 and prostaglandin F2α cause vasoconstriction.
- Histamine, bradykinin and prostaglandin E1 cause vasodilatation.
- Low O2 saturation, high CO2 saturation and acidosis also cause vasodilatation.
Temperature
- Heat causes cutaneous vasodilatation and increased flow, which predominantly bypasses capillary beds via AVAs.
The delay phenomenon
- Delay is any preoperative manoeuvre that results in increased flap survival.
- Historical examples include Tagliacozzi’s nasal reconstruction described in the 16th century.
- Involves elevation of a bipedicled flap with length : breadth ratio of 2:1.
- The flap can be considered as two 1:1 flaps.
- Cotton lint is placed under the flap, preventing its reattachment.
- Two weeks later, one end of the flap is detached from the arm and attached to the nose.
- A flap of these dimensions transferred without a delay procedure would have a significant chance of distal necrosis.
- Involves elevation of a bipedicled flap with length : breadth ratio of 2:1.
- Delay is occasionally used for pedicled TRAM breast reconstruction.
- The DIEA is ligated two weeks prior to flap transfer.
- The mechanism of delay remains incompletely understood.
- These theories have been proposed to explain the delay phenomenon:
Increased axiality of blood flow
- Removal of blood flow from the periphery of a random flap promotes development of an axial blood supply from its base.
- Axial flaps have improved survival compared to random flaps.
Tolerance to ischaemia
- Cells become accustomed to hypoxia after the initial delay procedure.
- Less tissue necrosis therefore occurs after the second operation.
Sympathectomy vasodilatation theory
- Dividing sympathetic fibres at the borders of a flap results in vasodilatation and improved blood supply.
- But why, if sympathectomy is immediate, does the delay phenomenon only begin to appear at 48 hours, and why does it take 2 weeks for maximum effect?
Intraflap shunting hypothesis
- Postulates that sympathectomy dilates AVAs, resulting in an increase in nonnutritive blood flow bypassing the capillary bed.
- A greater length of flap will survive at the second stage as there are fewer sympathetic fibres to cut and therefore less of a reduction in nutritive blood flow.
Hyperadrenergic state
- Surgery results in increased tissue concentrations of vasoconstrictors, such as epinephrine and norepinephrine.
- After the initial delay procedure, the resultant reduction in blood supply is not sufficient to produce tissue necrosis.
- The level of vasoconstrictor substances returns to normal before the second procedure.
- The second procedure produces another rise in the concentration of vasoconstrictor substances.
- This rise is said to be smaller than it would be if the flap were elevated without a prior delay.
- The flap is therefore less likely to undergo distal necrosis after a delay procedure.
- After the initial delay procedure, the resultant reduction in blood supply is not sufficient to produce tissue necrosis.
Unifying theory
- Described by Pearl in 1981; incorporates elements of all these theories.
Classification of flaps
- Flaps can be classified by the five ‘C’s:
- Circulation
- Composition
- Contiguity
- Contour
- Conditioning.
- Circulation
Circulation
- Can be further subcategorised into:
- Random
- Axial (direct, fasciocutaneous, musculocutaneous, or venous).
- Random
Random flaps
- No directional blood supply; not based on a named vessel.
- These include most local flaps on the face.
- Should have a maximum length : breadth ratio of 1:1 in the lower extremity, as it has a relatively poor blood supply.
- Can be up to 6:1 in the face, as it has a good blood supply.
Axial flaps
Direct
- Contain a named artery running in subcutaneous tissue along the axis of the flap.
- Examples include:
- Groin flap, based on superficial circumflex iliac vessels.
- Deltopectoral flap, based on perforating vessels of internal mammary artery.
- Groin flap, based on superficial circumflex iliac vessels.
- Both flaps can include a random segment in their distal portions after the artery peters out.
Fasciocutaneous
- Based on vessels running either within or near the fascia.
- The fasciocutaneous system predominates on the limbs.
- Fasciocutaneous flaps are classified by Cormack and Lamberty:
- The fasciocutaneous system predominates on the limbs.
Type A
- Dependent on multiple non-named fasciocutaneous vessels that enter the base of the flap.
- Lower leg ‘super flaps’ described by Pontén are examples of type A flaps.
- Their dimensions vastly exceed the 1:1 ratios recommended.
Type B
- Based on a single fasciocutaneous vessel, which runs along the axis of the flap.
- Examples include scapular/parascapular flap, and perforator-based fasciocutaneous flaps of the lower leg.
Type C
- Supplied by multiple small perforating vessels, which reach the flap from a deep artery running along a fascial septum between muscles.
- Examples include radial forearm flap (RFF) and lateral arm flap.
Type C flaps with bone
- Osteofasciocutaneous flaps, originally classified as type D.
- Examples include:
- RFF raised with a segment of radius; lateral arm flap raised with a segment of humerus.
- The Mathes and Nahai fasciocutaneous flap classification is slightly different:
Type A
- Direct cutaneous pedicle.
- Examples: groin, superficial inferior epigastric and dorsal metacarpal artery flaps.
Type B
- Septocutaneous pedicle.
- Examples: scapular and parascapular, lateral arm, posterior interosseous flap.
Type C
- Musculocutaneous pedicle.
- Examples: median forehead, nasolabial and (usually) anterolateral thigh flap.
Musculocutaneous
- Flaps based on perforators that reach the skin through the muscle.
- The musculocutaneous system predominates on the torso.
- Muscle and musculocutaneous flaps were classified by Mathes and Nahai in 1981:
Type I
- Single vascular pedicle.
- Examples: gastrocnemius, tensor fasciae latae (TFL), abductor digiti minimi.
- Good flaps for transfer—the whole muscle is supplied by a single pedicle.
- Examples: gastrocnemius, tensor fasciae latae (TFL), abductor digiti minimi.
Type II
- Dominant pedicle(s) and other minor pedicle(s).
- Examples: trapezius, soleus, gracilis.
- Good flaps for transfer—can be based on the dominant pedicle after the minor pedicle(s) are ligated.
- Circulation via minor pedicles alone is not reliable.
Type III
- Two dominant pedicles, each arising from a separate regional artery or opposite sides of the muscle.
- Examples: rectus abdominis, pectoralis minor, gluteus maximus.
- Useful muscles for transfer—can be based on either pedicle.
Type IV
- Multiple segmental pedicles.
- Examples: sartorius, tibialis anterior, long flexors and extensors of the toes.
- Seldom used for transfer—each pedicle supplies only a small portion of muscle.
Type V
- One dominant pedicle and secondary segmental pedicles.
- Examples: latissimus dorsi, pectoralis major.
- Useful flaps—can be based on either the dominant pedicle or secondary segmental pedicles.
Venous
- Based on venous, rather than arterial, pedicles.
- In fact, many venous pedicles have small arteries running alongside them.
- The mechanism of perfusion is not completely understood.
- Example: saphenous flap, based on long saphenous vein.
- Used to reconstruct defects around the knee.
- Venous flaps are classified by Thatte and Thatte:
Type 1
- Single venous pedicle.
Type 2
- Venous flow-through flaps, supplied by a vein that enters one side of the flap and exits from the other.
Type 3
- Arterialised through a proximal arteriovenous anastomosis and drained by distal veins.
- Venous flaps tend to become congested post-operatively.
- Survival is inconsistent; they have therefore not been universally accepted.
- Modifying the type 3 arterialised venous flap by restricting direct arteriovenous shunting can improve survival rates by redistributing blood to the periphery of the flap.
- Venous flaps tend to become congested post-operatively.
Composition
- Flaps can be classified by their composition as:
- Cutaneous
- Fasciocutaneous
- Fascial
- Musculocutaneous
- Muscle only
- Osseocutaneous
- Osseous.
- Cutaneous
Contiguity
- Flaps can be classified as:
- Local flaps
- Composed of tissue adjacent to the defect.
- Regional flaps
- Composed of tissue from the same region of the body as the defect, e.g. head and neck, upper limb.
- Distant flaps
- Pedicled distant flaps come from a distant part of the body to which they remain attached.
- Free flaps are completely detached from the body and anastomosed to recipient vessels close to the defect.
- Pedicled distant flaps come from a distant part of the body to which they remain attached.
- Local flaps
Contour
- Flaps can be classified by the way they are transferred into the defect:
Advancement
- Stretching the flap
- Excision of Burow triangles at the flap’s base
- V-Y advancement
- Z-plasty at its base
- Careful scoring of the undersurface
- Combinations of the above.
Transposition
- The flap is moved into an adjacent defect, leaving a secondary defect that must be closed by another method.
Rotation
- The flap is rotated into the defect.
- Classically, rotation flaps are designed to allow closure of the donor defect.
- In reality, many flaps have elements of transposition and rotation, and may be best described as pivot flaps.
- Classically, rotation flaps are designed to allow closure of the donor defect.
Interpolation
- The flap is moved into a defect either under or above an intervening bridge of tissue.
Crane principle
- This aims to transform an ungraftable bed into one that will accept a skin graft.
- At the first stage, a flap is placed into the defect.
- After sufficient time to allow vascular ingrowth into the flap from the recipient site, a superficial part of the flap is replaced in its original position.
- This leaves a segment of subcutaneous tissue in the defect, which can now accept a skin graft.
Conditioning
- This involves delaying the flap, discussed in ‘Blood supply to the skin’.
Geometry of local flaps
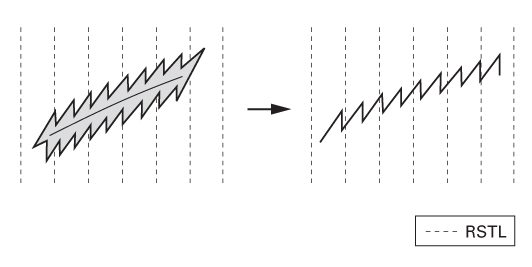
Orientation of elective incisions
- In the 19th century, Langer showed that circular awl wounds produced elliptical defects in cadaver skin.
- He believed this occurred because skin tension along the longitudinal axis of the ellipse exceeded that along the transverse axis.
- Borges has provided over 36 descriptive terms for skin lines, including:
- Relaxed skin tension lines (RSTLs)—these are parallel to natural skin wrinkles (rhytids) and tend to be perpendicular to the fibres of underlying muscles.
- Lines of maximum extensibility (LME)—these lie perpendicular to RSTLs and parallel to the fibres of underlying muscles.
- Relaxed skin tension lines (RSTLs)—these are parallel to natural skin wrinkles (rhytids) and tend to be perpendicular to the fibres of underlying muscles.
- The best orientation of an incision can be judged by a number of methods:
- Knowledge of the direction of pull of underlying muscles.
- Making the incision parallel to any rhytids or RSTLs.
- Making the incision perpendicular to LMEs.
- Making the incision parallel to the direction of hair growth.
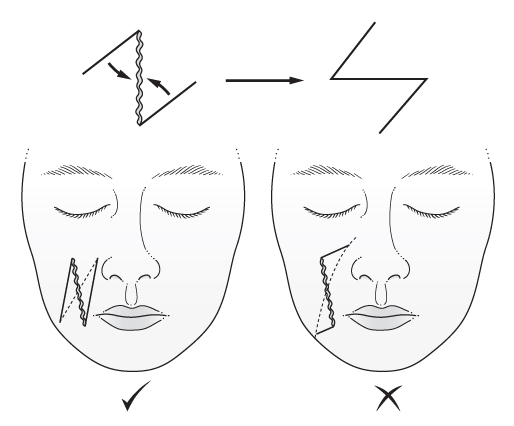
- ‘The pinch test’—if skin either side of the planned incision is pinched, it forms a transverse fold without distortion if it is orientated correctly; if a sigmoid-shaped fold forms, it is orientated incorrectly.
- Knowledge of the direction of pull of underlying muscles.
Plasty techniques
Z-plasty
- Involves transposition of two adjacent triangular-shaped flaps.
- Can be used to:
- Increase the length of an area of tissue or scar
- Break up a straight-line scar
- Realign a scar.
- The degree of elongation of the longitudinal axis of the Z-plasty is directly related to the angles of its constituent flaps.
- 30° → 25% elongation
- 45° → 50% elongation
- 60° → 75% elongation
- 75° → 100% elongation
- 90° → 125% elongation.
- The amount of elongation can be worked out by starting at 30° and 25% and adding 15° and 25% to each of the figures.
- Gains in length are estimates; true values depend on local tissue elasticity and tension.
- Flaps with 60° angles are most commonly used as they lengthen without undue tension.
- The angles of the two flaps need not be equal and can be designed to suit local tissue requirements.
- However, all three limbs should be of the same length.
- When designing a Z-plasty to realign a scar:
- Mark the desired direction of the new scar.
- Draw the central limb of the Z-plasty along the original scar.
- Draw the lateral limbs of the Z-plasty from the ends of the central limb, to the line drawn in (1).
- Two patterns will be available, one with a wide angle at the apex of the flaps, the other with a narrow angle.
- Select the pattern with the narrower angle as these flaps transpose better.
- Increase the length of an area of tissue or scar
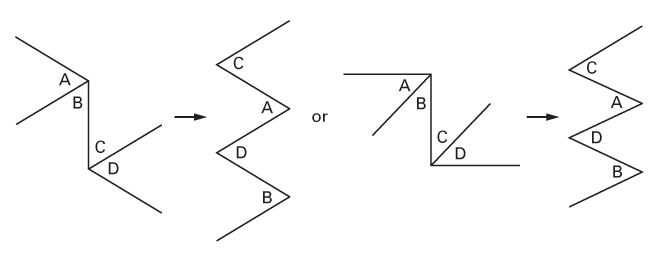
The four-flap plasty
- It is, in effect, two interdependent Z-plasties.
- Can be designed with different angles.
- The two outer flaps become the inner flaps after transposition.
- The two inner flaps become the outer flaps after transposition.
- The flaps, originally in an ‘ABCD’ configuration, end as ‘CADB’ (CADBury).
- Can be designed with different angles.
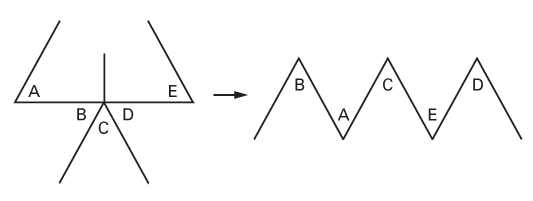
The five-flap plasty
- Because of its appearance, this is also called a jumping-man flap.
- Used to release first web space contractures and epicanthal folds.
- It is, in effect, two opposing Z-plasties with a V-Y advancement in the center.
- The flaps, originally in an ‘ABCDE’ configuration, end as ‘BACED’.
The W-plasty
- Used to break up the line of a scar and improve its aesthetics.
- Unlike the Z-plasty, it does not lengthen tissue.
- If possible, one of the limbs of the W-plasty should lie parallel to the RSTLs so that half of the resultant scar will lie parallel to them.
- Using a template helps ensure each wound edge interdigitates easily.
- The technique discards normal tissue, which may be a disadvantage in certain areas.
Local flaps
- Advancement flaps (simple, modified, V-Y, keystone, bipedicled).
- Pivot flaps (transposition, interpolation, rotation, bilobed).
Advancement flaps
Simple
- Rely on skin elasticity.
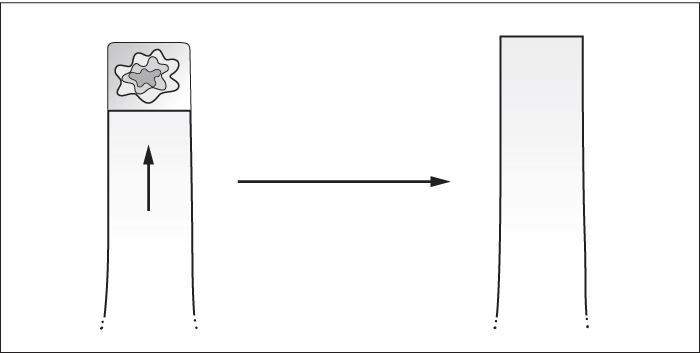
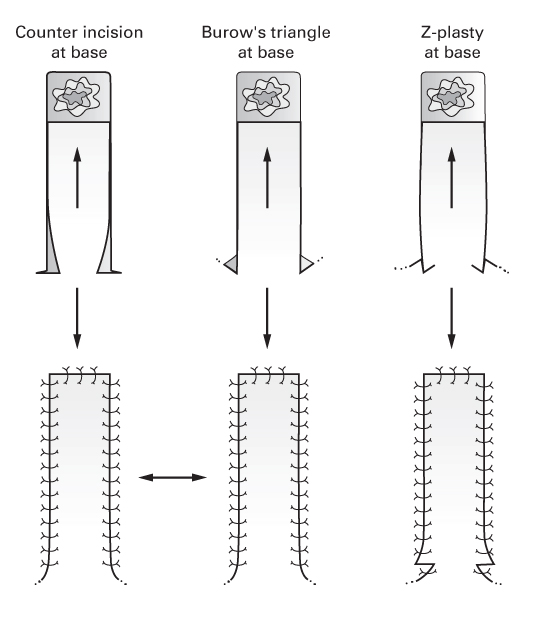
Modified
- Incorporate one of the following at the flap’s base to increase advancement:
- Counter incision
- Excision of Burow’s triangle
- Z-plasty.
- Counter incision
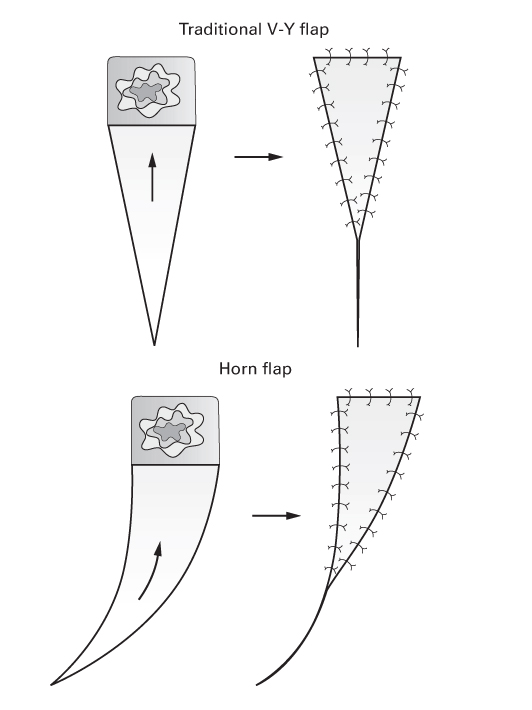
V-Y
- These are incised along their cutaneous borders.
- Their blood supply comes from deep tissue through a subcutaneous pedicle.
- Horn flaps and oblique V-Y flaps are modifications of the original V-Y.
Keystone
- Trapezoidal flaps used to close elliptical defects.
- Essentially two V-Y flaps end-to-side.
- Designed to straddle longitudinal structures, e.g. superficial nerves and veins, which are incorporated into the flap.
- Blunt dissection to deep fascia preserves perforators and subcutaneous veins.
- The lateral deep fascial margin can be incised for increased mobilisation.
- The extremes of the donor site are closed as V-Y advancements, which produces transverse laxity in the flap.
- Essentially two V-Y flaps end-to-side.
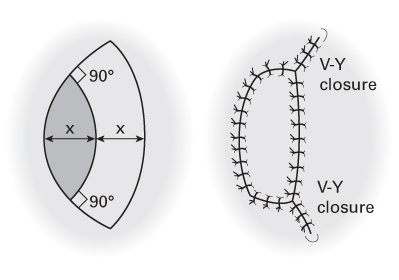
Bipedicled
- Receive blood supply from both ends.
- Less prone to necrosis than flaps of similar dimensions attached only at one end.
- Example: von Langenbeck mucoperiosteal flap, used to repair cleft palates.
- Bipedicled flaps are designed to curve parallel with the defect.
- This permits flap transposition with less tension.
- Less prone to necrosis than flaps of similar dimensions attached only at one end.
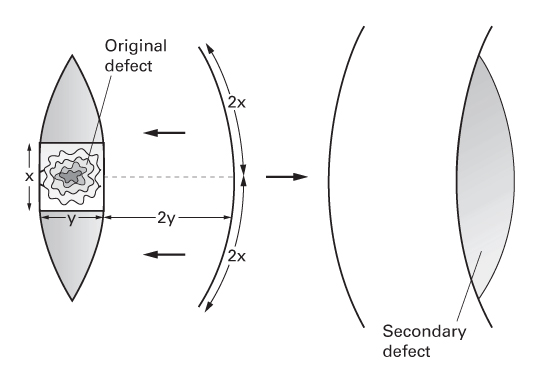
Pivot flaps
Transposition flaps
- Transposed into the defect, leaving a donor site that is closed by some other means (often a skin graft).
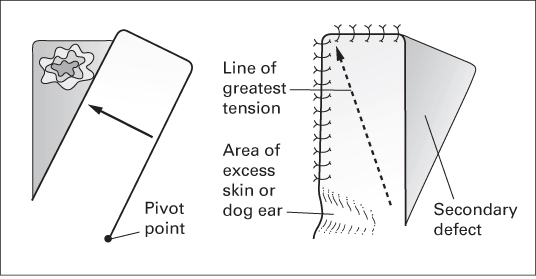
Transposition flaps with direct closure of donor site
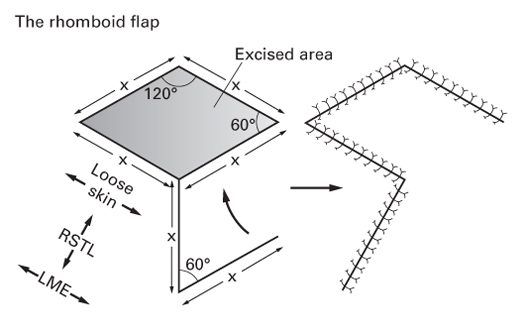
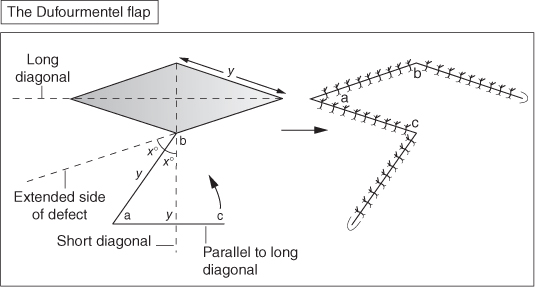
- Include the rhomboid flap (Limberg flap) and Dufourmentel flap.
- These are similar in concept but vary in geometry.
- Both are designed to leave the donor site scar parallel to RSTLs.
The rhomboid flap
The Dufourmentel flap
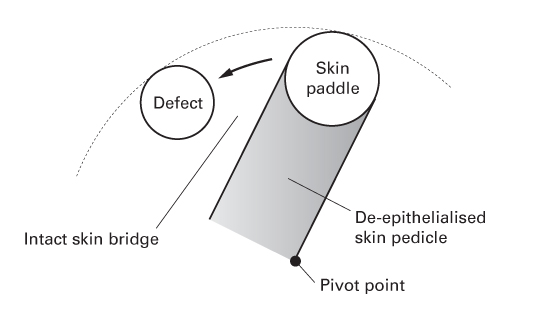
Interpolation flaps
- Flaps raised from local, but not adjacent, skin.
- The pedicle is passed either over or under an intervening skin bridge.
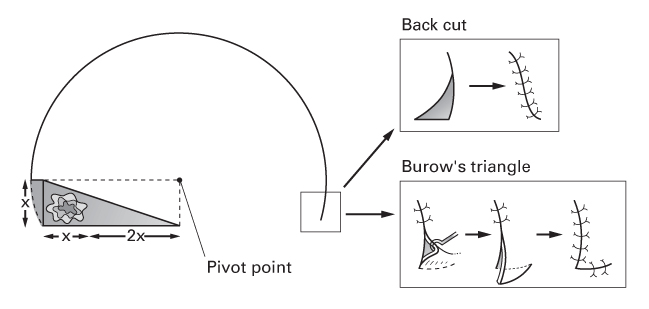
Rotation flaps
- These large flaps rotate tissue into the defect.
- Tissue redistribution usually permits direct closure of the donor site.
- Flap circumference should be 5–8 times the width of the defect.
- These are used on the scalp for hair-bearing reconstruction.
- The back cut at the flap’s base can be directed towards or away from the defect.
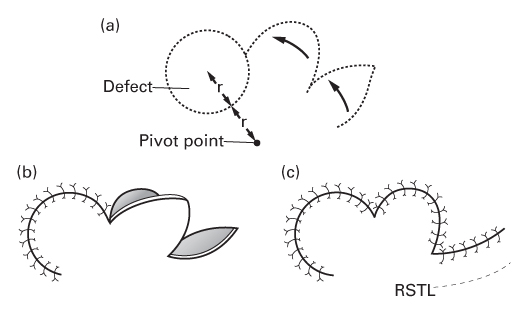
The bilobed flap
- Various designs have been described.
- Consists of two transposition flaps.
- The first flap is transposed into the original defect.
- The second flap is transposed into the secondary defect—the donor site of the first flap.
- The tertiary defect at the donor site of the second flap closes directly.
- This suture line is designed to lie parallel to RSTLs.
- Esser, who first described the flap, put the first flap at 90° to the defect and the second flap at 90° to the first flap.
- Zitelli modified these angles to 45° each, resulting in smaller dog ears.
- Consists of two transposition flaps.
Wound healing and skin grafts
- Healing by primary intention
- Skin edges are directly opposed.
- Healing is normally good, with minimal scar formation.
- Skin edges are directly opposed.
- Healing by secondary intention
- The wound is left open to heal by a combination of granulation tissue formation, contraction and epithelialisation.
- More inflammation and proliferation occurs compared to primary healing.
- Healing by tertiary intention
- Wounds are initially left open, then closed as a secondary procedure.
- The wound is left open to heal by a combination of granulation tissue formation, contraction and epithelialisation.
Phases of wound healing
- Haemostasis
- Inflammation
- Proliferation
- Remodelling.
Haemostasis
- Vasoconstriction occurs immediately after vessel division due to release of thromboxanes and prostaglandins from damaged cells.
- Platelets bind to exposed collagen, forming a platelet plug.
- Platelet degranulation activates more platelets and increases their affinity to bind fibrinogen.
- Involves modification of membrane glycoprotein IIb/IIIa (blocked by clopidogrel).
- Platelet activating factor (PAF), von Willebrand factor (vWF) and thromboxane A2 stimulate conversion of fibrinogen to fibrin.
- This propagates formation of thrombus.
- Thrombus is initially pale when it contains platelets alone (white thrombus).
- As red blood cells are trapped, the thrombus becomes darker (red thrombus).
Inflammation
- Occurs in the first 2–3 days after injury.
- Stimulated by physical injury, antigen–antibody reaction or infection.
- Platelets release growth factors, e.g. platelet-derived growth factor (PDGF).
- Also release proinflammatory factors, e.g. serotonin, bradykinin, prostaglandins, thromboxanes and histamine.
- These increase cell proliferation and migration.
- Also release proinflammatory factors, e.g. serotonin, bradykinin, prostaglandins, thromboxanes and histamine.
- Endothelial cells swell, causing vasodilatation and allowing egress of polymorphonuclear neutrophils (PMNs) and monocytes into the tissue.
- T lymphocytes migrate into the wound under the influence of interleukin-1.
- Lymphocytes secrete various cytokines, including epidermal growth factor and basic fibroblast growth factor (bFGF).
- They also play a role in cellular immunity and antibody production.
Proliferation
- Begins on the 2nd or 3rd day and lasts for 2–4 weeks.
- Monocytes mature into macrophages that release PDGF and transforming growth factor-β (TGF-β), which are chemoattractant to fibroblasts.
- Fibroblasts, usually located in perivascular tissue, migrate along fibrin networks into the wound.
- Fibroblasts secrete GAGs to produce ground substance, and then produce collagen and elastin.
- Initially, type III collagen is produced to increase the strength of the wound.
- Some fibroblasts differentiate into myofibroblasts and effect wound contraction.
- Angiogenesis occurs concurrently to supply oxygen and nutrients to the wound.
- Endothelial stem cells from blood vessels migrate through extracellular matrix.
- Attracted to the wound by angiogenic factors, thrombus and local hypoxia.
- Zinc-dependent matrix metalloproteinases aid cell migration through tissues.
Remodelling
- Begins 2–4 weeks after injury and can last a year or longer.
- During remodelling there is no net increase in collagen (collagen homeostasis).
- Type III collagen is replaced by the stronger type I collagen.
- Collagen fibres, initially laid down haphazardly, are arranged in a more organised manner.
- The wound’s tensile strength approaches 50% of normal by 3 months; eventually becomes 80% as strong.
- The extensive capillary network is no longer required and is removed by apoptosis, leaving a pale collagen scar.
Abnormal scars
- Classified as either hypertrophic or keloid.
- Keloids extend beyond the original wound margins.
- Hypertrophic scars are limited to original wound margins; commoner than keloids.
- Keloids extend beyond the original wound margins.
- Increased numbers of mast cells in abnormal scars may account for the pruritus experienced by some patients.
Hypertrophic scars
- Usually occurs within 8 weeks of wounding.
- Grow rapidly for up to 6 months before gradually regressing to a flat, asymptomatic scar.
- This may take a few years.
- Typically form at locations under tension, e.g. shoulders, neck, presternal area, knees, ankles.
- Microscopy shows well-organised type III collagen bundles with nodules containing myofibroblasts.
Keloid scars
- Dark-skinned individuals are more prone to keloid scars.
- There is often a family history.
- May develop at any point up to several years after minor injuries.
- Typically persist for long periods of time and do not regress spontaneously.
- Pain and hypersensitivity are associated more with keloids than hypertrophic scars.
- Commonly form on anterior chest, shoulders, earlobes, upper arms and cheeks.
- Excision typically results in recurrence.
- Microscopy shows poorly organised type I and III collagen bundles with few myofibroblasts.
- Expression of proliferating cell nuclear antigen (PCNA) and p53 is upregulated.
Epithelial repair
- If the epidermal basement membrane is not breached, epithelial cells are replaced by upward migration of keratinocytes as in uninjured skin.
- If the basement membrane is breached, re-epithelialisation must occur from the wound margins and, if present and intact, from epidermal appendages.
- Re-establishing epithelial continuity consists of these four phases:
Mobilisation
- Epithelial cells at the wound edges elongate, flatten and form pseudopodia.
- They detach from neighbouring cells and basement membrane.
Migration
- Decreased contact inhibition promotes cell migration.
- Epithelial cells climb over one another to migrate.
- As cells migrate, epithelial cells at the wound edge proliferate to replace them.
- Cells migrate until they meet those from the opposite wound edge.
- At this point, contact inhibition is reinstituted and migration ceases.
Mitosis
- Epithelial cells proliferate once they have covered the wound.
- They secrete proteins to form a new basement membrane.
- Cells reverse the morphological changes required for migration.
- Desmosomes and hemidesmosomes are re-established to anchor themselves to the basement membrane and to each other.
- This new epithelial cell layer forms a stratum germinativum and undergoes mitosis as in normal skin.
Cellular differentiation
- The normal structure of stratified squamous epithelium is re-established.
Collagen
- Constitutes approximately 30% of total body protein.
- Formed by hydroxylation of amino acids lysine and proline by enzymes that require vitamin C as a cofactor.
- Procollagen is initially formed within the cell.
- Procollagen is transformed into tropocollagen after it is excreted from the cell.
- Fully formed collagen has a complex structure.
- Consists of three polypeptide chains wound in a left-handed helix.
- These three chains are further wound in a right-handed coil to form the basic tropocollagen unit.
- Consists of three polypeptide chains wound in a left-handed helix.
- Collagen formation is inhibited by colchicine, penicillamine, steroids and deficiencies of vitamin C and iron.
- Cortisol stimulates degradation of skin collagen.
- Thus far, 28 types of collagen have been identified.
- Each type shares the same basic structure but differs in the relative composition of hydroxylysine and hydroxyproline, and in the degree of cross-linking between chains.
- The five most common types are:
- Type I: predominant in mature skin, bone and tendon.
- Type II: present in hyaline cartilage and cornea.
- Type III: present in healing tissue, particularly fetal wounds.
- Type IV: predominant constituent of basement membranes.
- Type V: similar to type IV. Also found in hair and placenta.
- The ratio of type I collagen to type III collagen in normal skin is 5:1.
- Hypertrophic and immature scars contain ratios of 2:1 or less.
- 90% of total body collagen is type I.
- Cortisol stimulates degradation of skin collagen.
The macrophage
- Derived from mononuclear leukocytes.
- Debrides tissue and removes micro-organisms.
- Co-ordinates angiogenesis and fibroblast activity by releasing growth factors:
- PDGF, FGF 1 and 2, tumour necrosis factor alpha (TNF-α) and TGF-β.
- Essential for normal wound healing.
- Wounds depleted of macrophages heal slowly.
The myofibroblast
- First identified by Gabbiani in 1971.
- Differs from a fibroblast—contains cytoplasmic filaments of α-smooth muscle actin, which are also found in smooth muscle.
- Actin fibres within myofibroblasts are thought to be responsible for wound contraction.
- The number of myofibroblasts within a wound is proportional to its contraction.
- Increased numbers have been found in the fascia of Dupuytren’s disease.
- Thought to be responsible for the abnormal contraction of this tissue.
TGF-β
- Macrophages, fibroblasts, platelets, keratinocytes and endothelial cells secrete this growth factor.
- Believed to play a central role in wound healing:
- Chemoattraction of fibroblasts and macrophages
- Induction of angiogenesis
- Stimulation of extracellular matrix deposition
- Keratinocyte proliferation.
- Chemoattraction of fibroblasts and macrophages
- Three isoforms have been identified:
- Types 1 and 2 promote wound healing and scarring; upregulated in keloids.
- Type 3 decreases wound healing and scarring—may have a role as an antiscarring agent.
- Types 1 and 2 promote wound healing and scarring; upregulated in keloids.
- Fetal wounds have higher levels of TGF-β3 than adult wounds.
- TGF-β3 is thought to antagonise TGF-β1 and 2.
- May be one factor responsible for decreased inflammation and improved scarring observed in fetal tissue.
- TGF-β3 is thought to antagonise TGF-β1 and 2.
Factors affecting healing
- Systemic
- Congenital
- Acquired
- Congenital
- Local.
Systemic factors: congenital
Pseudoxanthoma elasticum
- Autosomal recessive.
- Characterised by increased collagen degradation and mineralisation.
- Skin is pebbled and extremely lax.
- Most have premature arteriosclerosis in their 30s.
Ehlers–Danlos syndrome
- Heterogeneous collection of connective tissue disorders.
- Most are autosomal dominant.
- Results from defects in synthesis, structure or cross-linking of collagen.
- Clinical features:
- Hypermobile fingers
- Hyperextensible skin
- Fragile connective tissues.
- Hypermobile fingers
- Surgery is avoided if possible—wound healing is poor.
Cutis laxa
- Presents in the neonatal period.
- Skin is abnormally lax.
- Patients have inelastic, coarsely textured, drooping skin.
Progeria
- Characterised by premature ageing.
- Clinical features:
- Growth retardation
- Wrinkled skin
- Baldness
- Atherosclerosis.
- Growth retardation
Werner syndrome
- Autosomal recessive.
- Skin changes similar to scleroderma.
- Elective surgery avoided whenever possible—healing is poor.
Epidermolysis bullosa
- Heterogeneous collection of separate conditions.
- Skin is very susceptible to mechanical stress.
- Blistering may occur after minor trauma (Nikolsky sign).
- The most severe subtype, dermolytic bullous dermatosis (DBD), results in hand fibrosis and syndactyly—the ‘mitten hand’ deformity.
- Patients may develop squamous cell carcinoma in areas of chronic erosion.
- Skin is very susceptible to mechanical stress.
Systemic factors: acquired
Nutrition
- Vitamin A involved in collagen cross-linking; deficiency delays wound healing.
- Vitamin C required for collagen synthesis.
- Vitamin E acts as a membrane stabiliser; deficiency may inhibit healing.
- Zinc, copper and selenium are important cofactors for many enzymes; administration accelerates healing in deficient states.
- Hypoalbuminaemia is associated with poor healing.
Pharmacological
- Steroids decrease inflammation and subsequent wound healing.
- Cytotoxics damage basal keratinocytes.
- Non-steroidal anti-inflammatory drugs (NSAIDs) decrease collagen synthesis.
- Anti-TNF-α drugs used in rheumatoid may increase post-operative wound complications.
Endocrine abnormalities
- Diabetics often have delayed wound healing; this is multifactorial.
- Untreated hypothyroidism is associated with slow healing.
Age
- Cell multiplication rates decrease with age.
- All stages of healing are therefore protracted.
- Healed wounds have decreased tensile strength in the elderly.
Smoking
- Nicotine is a sympathomimetic that causes vasoconstriction and consequently decreases tissue perfusion.
- Carbon monoxide in cigarette smoke decreases oxygen-carrying capacity of haemoglobin.
- Hydrogen cyanide in cigarette smoke poisons intracellular oxidative metabolism pathways.
Local factors
Infection
- Subclinical wound infection can impair wound healing.
- Wounds with >105 organisms per gram of tissue are considered infected and are unlikely to heal without further treatment.
Radiation
- Causes endothelial cell, capillary and arteriole damage.
- Irradiated fibroblasts secrete less collagen and extracellular matrix.
- Lymphatics are also damaged, resulting in oedema and an increased infection risk.
- Irradiated fibroblasts secrete less collagen and extracellular matrix.
Blood supply
- Decreased tissue perfusion results in decreased wound oxygenation.
- Fibroblasts are oxygen-sensitive and their function is reduced in hypoxic tissue.
- Reduced oxygen delivery results from decreases in:
- Inspired oxygen concentration
- Oxygen transfer to haemoglobin
- Haemoglobin concentration
- Tissue perfusion.
- Inspired oxygen concentration
- Decreased oxygen delivery to tissue reduces:
- Collagen formation
- Extracellular matrix deposition
- Angiogenesis
- Epithelialisation.
- Collagen formation
- Hyperbaric oxygen increases inspired oxygen concentration but its effectiveness relies on good tissue perfusion.
Trauma
- The delicate neoepidermis of healing wounds is disrupted by trauma.
Neural supply
- There is evidence that wounds in denervated tissue heal slowly.
- May contribute to delayed healing observed in some pressure sores, and in patients with diabetes and leprosy.
- Mechanisms are poorly understood, but may be related to levels of chemoattractant neuropeptides in the wound.
Fetal wound healing
- Tissue healing in the first 6 months of fetal life occurs by regeneration rather than scarring.
- Regenerative healing is characterised by absence of scarring.
- Normal dermal structures such as hair follicles form normally.
- Regenerative healing differs from adult healing:
- Reduced inflammation.
- Reduced platelet aggregation and degranulation.
- Reduced angiogenesis.
- Epithelialisation is more rapid.
- Virtually no myofibroblasts and no wound contraction.
- Collagen deposition is rapid, organised and not excessive.
- More type III than type I collagen is laid down.
- The wound contains more water and hyaluronic acid.
- Reduced inflammation.
- Relative proportions of TGF-β isoforms may be responsible for some of these differences.
Skin grafts
- Skin grafts are either full or split thickness.
- Split-skin grafts contain the epidermis and a variable amount of dermis.
- Usually harvested from thigh or buttock.
- Full-thickness skin grafts contain the entire epidermis and dermis.
- Usually harvested from areas that allow direct closure of the donor defect.
- Primary contraction is the immediate recoil observed in freshly harvested skin.
- Due to elastin in the dermis.
- Secondary contracture occurs after the graft has healed.
- Due to myofibroblast activity.
- The thicker the graft, the greater the degree of primary contraction.
- The thinner the graft, the greater the degree of secondary contracture.
- Split-skin grafts contain the epidermis and a variable amount of dermis.
Mechanisms
- Skin grafts heal in four phases:
Adherence
- Fibrin bonds form immediately on applying skin graft to a suitable bed.
Serum imbibition
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






