Foam Sclerotherapy
 INTRODUCTION
INTRODUCTION
Sclerotherapy with a foamed solution has become the standard treatment of many varicosities, perforating veins, reticular veins, and larger telangiectasias or venulectasias (1.0-2 mm). Although any detergent solution may be agitated with air and foamed, the two most popular solutions that are typically foamed are the detergent solutions, sodium tetradecyl sulfate (STS, sold in the United States as Sotradecol®; Bioniche Pharma, USA, LLC, Lake Forest, IL) and polidocanol (POL, sold in the United States as Asclera®; Chemische Fabrik Kreussler & Co., GmbH, Wiesbaden, Germany). Currently, to agitate and foam these U.S. Food and Drug administration (FDA)-cleared sclerosants is an off-label use in the United States. Foaming of solutions cannot be discussed or suggested by the companies manufacture or distribute the solutions in the United States because of the off-label use status.
Foamed sclerosant completely fills and displaces the blood in the vein, maximizing endothelial cell injury and subsequent fibrosis. Most phlebologists agree that this more effective form of sclerotherapy usually requires fewer treatment sessions than liquid sclerotherapy. Foam sclerotherapy has been thought to be as effective as surgery for larger branch varicosities.1
 HISTORY
HISTORY
Sclerotherapy with foamed sclerosant is not a new concept. In fact, physicians have been experimenting with foam injections since the 1940s. Within this longer than originally thought history of foam sclerotherapy, there have been several key contributions along the way.2 Until recently, foam techniques were not uniformly reproducible or particularly effective and did not garner mainstream acceptance. In 1995, a re-emergence of the foam sclerotherapy concept began with the work of Dr. Juan Cabrera, a Spanish vascular surgeon.3 He experimented with the use of detergent sclerosant (POL) mixed under high speeds with CO2 gas to create a therapeutic sclerotherapy foam. He studied high-volume foam injections into patients with incompetent great saphenous veins (GSVs) in order to replace surgical ligation. The resurgence of foam sclerotherapy was continued by Alain Monfreux in 1997 and Javier Garcia-Mingo in 1999.4,5 Monfreux developed a low-pressure foaming technique widely utilized in France and Garcia-Mingo described a sterilizable medical device for the preparation of a standardized foam. In the year 2000, sclerosant foam was introduced and by 2001 was widely accepted into the mainstream practice of phlebology by the work of an Italian phlebologist, Dr. Lorenzo Tessari.6,7 The foaming technique he developed was simple, inexpensive, and easily reproducible.
 TECHNIQUES OF SCLEROTHERAPY FOAM PREPARATION
TECHNIQUES OF SCLEROTHERAPY FOAM PREPARATION
Currently, the Tessari and the closely related Frullini and double syringe system (DDS) techniques are the most utilized techniques for the creation of foam sclerotherapy. In Tessari’s method, he connected two 3-cc Luer lock syringes with a disposable three-way stopcock (Figure 19-1). One syringe is filled with air and the other is filled with a detergent type of sclerosant. He found the optimal ratio of solution to air to be 1:4. For example, our practice uses 0.5 cc of liquid STS or 0.5 cc of liquid POL in the first syringe with 2 cc of air in the second syringe. The contents of the syringes are shifted back and forth quickly 10-20 times and the turbulent flow generates the foam. Tessari’s colleague, Dr. Alessandro Frullini, advocated a similar technique but created the foam using a two-way connector instead of a three-way stopcock.8 Frullini’s method has also been adapted into the DDS. The DDS kit consists of two sterile 10-cc plastic syringes (one with a rubber plunger and one without), a 0.2-μm filter to sterilize the air, and a two-way connector.
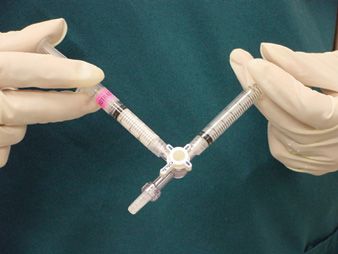
![]() FIGURE 19-1 The Tessari technique utilizes a three-way stopcock to connect two syringes, one filled partially with sclerosing solution and the other partially with air.
FIGURE 19-1 The Tessari technique utilizes a three-way stopcock to connect two syringes, one filled partially with sclerosing solution and the other partially with air.
 TYPES OF FOAM
TYPES OF FOAM
There are many variables when examining the types of sclerosant foam. These include the type and concentration of sclerosing agent, type of gas mixture, ratio of liquid to gas, method of preparation, and bubble size. Currently, only two types of sclerosants are widely used, the detergent sclerosants, STS or POL. The type of gas most commonly used is room air. But other types of gases utilized include sterile air, CO2, and other various proprietary mixtures of gas. The ratio of liquid to gas most commonly employed is 1:4. A 2011 study found that the halflife of CO2 foam did not vary according to the concentration of sclerosant solution, although room air, O2, and a combination of CO2 and O2 did.9
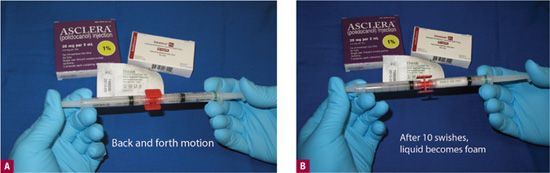
Foam may be prepared immediately before the injection, so termed as “extemporary” foam, or it may be manufactured. The only manufactured foam currently in development is Varisolve®(Provensis, Ltd., London, UK). Varisolve® is currently undergoing Phase III clinical trials in the United States. It is an aerosolized mixture of POL and CO2 gas and is not yet commercially available.10 Extemporaneous or immediate foam may be created by two syringes, as in the Tessari or Frullini method or through the use of an automated medical device, Turbofoam® (i2M Labs, Caen, France). The vast majority of phlebologists are using self-generated impromptu foam generated by the turbulence of room air between two syringes. The method that we use is the modified Tessari method using a luer-to-luer adapter and this is shown in Figures 19-2A and 19-2B.
![]() FIGURE 19-2 The modified Tessari technique utilizes a luer-to-luer lock adaptor to connect two syringes, one filled partially with sclerosing solution and the other partially with air. A. Immediate. B. After 10 strokes back and forth.
FIGURE 19-2 The modified Tessari technique utilizes a luer-to-luer lock adaptor to connect two syringes, one filled partially with sclerosing solution and the other partially with air. A. Immediate. B. After 10 strokes back and forth.
Foams may also be characterized by bubble size. For example, the bubble size in froth is greater than or equal to 1 mm, in foam greater than 100 microns, in minifoam less than 100 and greater than 50 microns, and in microfoam less than 50 microns. Microfoams are the most stable and the ones most commonly utilized. When mixing foam in a syringe with room air, there is a mixture of various sized bubbles that deteriorate within 60-90 s. As the bubbles dissipate, the solution goes back into the liquid phase. As a rule, the less the air volume compared to liquid, the worse the quality or stiffness of the foam and the faster the bubbles dissipate. Some physicians rarely use a 1:1 or 1:2 sclerosant-to-air ratio to generate a very weak or less viscous foam to inject telangiectasias.
 FOAM QUALITY
FOAM QUALITY
Once Frullini or Tessari foam has been generated, it should be used immediately. Most concentrations of foam degrade to half by approximately 90 s. When foam degrades, it separates back into liquid and air. This was confirmed by Rao and Goldman, who compared the foam half-life of various concentrations of STS and POL (Tables 19-1 and 19-2).11 Lai and Goldman also studied the foam quality in relation to the use of different commercially available syringe connectors.12 They concluded that the difference in foam quality was due to the silicone content in the barrel of the syringe.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree