I. ANATOMY
A. Definitions
1. Flap: Segment of tissue that is transferred with its own blood supply (in contrast to graft, which is revascularized from recipient bed).
2. Pedicle: Base of flap that contains blood supply.
3. Pedicled flap: Remains attached to native vascular supply
4. Free flap: Fully detached from vascular supply and reconnected to recipient vessels using microvascular technique.
B. Flap classification
1. Blood supply
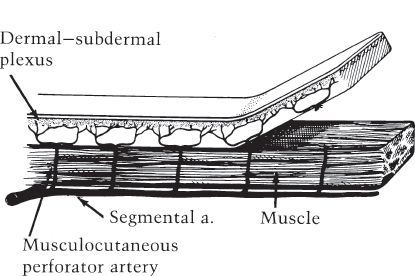
a. Random pattern flap: Raised without regard to any named blood supply, relying on blood flow through subdermal plexus (Fig. 4-1).
b. Axial flaps: Raised on dominant (named) arterial supply running along its long axis.
c. Perforator flap: Blood supply from perforator from dominant feeding vessel.
d. Reverse flow flaps: Dominant supply is divided, flap left to survive on intact distally based vessels that form connections to another blood supply system.
2. Location
a. Local flap: Shares side with the defect
b. Regional flap: In same region of the body as the defect, but does not share defect margin.
c. Distant flap: Not in the region of the defect, located in a different part of the body
3. Method of transfer
a. Advancement
b. Transposition
c. Rotation
d. Interpolation
e. Jumping
f. Free
Figure 4-1. Random pattern skin flap.
______________
*Denotes common in-service examination topics
a. Cutaneous flap
b. Fascia/fasciocutaneous flap
c. Muscle/musculocutaneous flap
d. Osseous/osteocutaneous/osteomusculocutaneous flap
e. Omental flap
C. Angiosomes
1. Definition: Composite unit of skin and deeper structures between skin and bone supplied by a source vessel.
2. Entire surface area of body composed of angiosomes.
3. Majority of flaps cover more than two angiosomes.
4. Neighboring angiosomes can be linked by true arterial anastomoses or by choke vessels (reduced caliber anastomoses) that dilate under certain circumstances, such as flap delay (see below).
5. Connections between angiosomes explain how flaps can support more than one angiosome area under certain conditions.
II. FLAP SELECTION
A. Reconstructive goals
1. Restore form and function to the defect
2. Minimize donor site morbidity
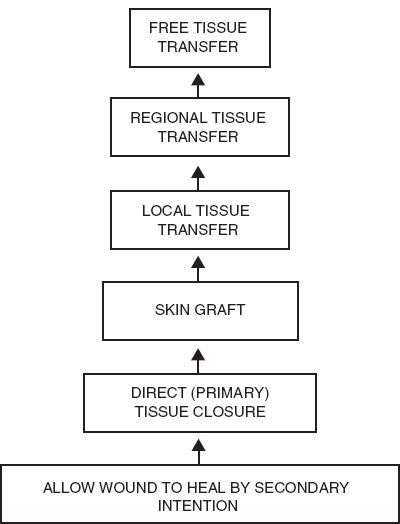
B. Reconstructive ladder (Fig. 4-2)
1. Systematic approach to facilitate decision-making for reconstruction of defects.
2. Least complicated technique chosen to address needs of the defect and reconstructive goals.
3. Ladder progresses from simple to complex options
a. Healing by secondary intention
b. Primary closure
c. Skin graft
d. Local flap
Figure 4-2. Reconstructive ladder.
f. Distant flap
g. Free flap
C. Reconstructive elevator
1. Often best solution is not simplest
2. Option is chosen that will give patient best aesthetic and functional result, often requiring a “jump” in the ladder (e.g., free flap may be the best first choice if superior result is unmatched by other options, even if simpler option can also be used).
3. In reality, this is the method in which flap selection is typically done.
D. Flap selection considerations
1. Goals of intervention
2. Shape, contour, and structural needs of reconstruction
3. Location of the defect
4. Size of the defect
5. Exposed and underlying structures
6. Viability of surrounding tissue (e.g., previous radiation, vascular disease, tissue necrosis)
7. Available donor sites
8. Effect on donor site: Morbidity, secondary defect, distortion of nearby structures (especially important in face)
9. Pedicle length and caliber
10. Technical demand
11. Patient expectations
12. Patient comorbidities
13. Anticipated risk for complications (infection, would healing complications, etc.)
14. Cost of care
III. CUTANEOUS FLAPS
A. Indications
1. Reconstruction of the local defect with similar, adjacent tissue
2. Need for full-thickness skin coverage of relatively less vascularized tissue (e.g., coverage of bone, tendon without periosteum/paratenon intact) for which skin graft is insufficient
B. Blood supply: Dependent on blood supply from fascial plexus (unless random pattern)
1. Direct cutaneous (axial) arteries
2. Septocutaneous arteries
3. Musculocutaneous arteries
4. Random pattern flaps
a. Designed on random vascular supply from subdermal plexus
b. *Size limited to length-to-width ratio ~2:1 in lower extremity and up to 4:1 in head and neck.
c. Ischemia expected when the recommended length-to-width ratio dimensions exceeded without flap delay.
d. Most (but not all) small local cutaneous flaps based on random blood supply
C. Method of transfer: Advancement, pivotal, or hinge
1. Advancement flaps: Moved by sliding or stretching flap toward the defect in one direction, requires skin laxity
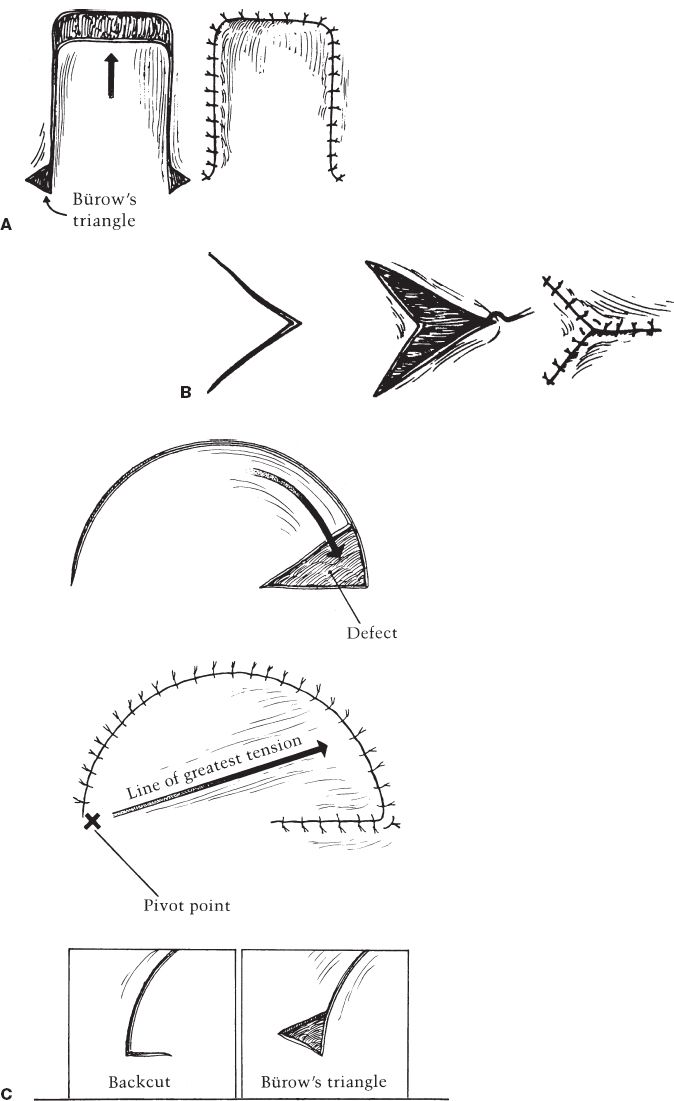
a. Single-pedicle advancement flap (Fig. 4-3A)
i. Raised as square or rectangle
ii. Undermined and advanced to fill defect sharing border with flap
iii. Bürow’s triangles made at base to facilitate advancement and closure (helps correct length discrepancy between skin surrounding wound and skin of flap margin)
b. Bipedicle advancement flap
i. Incision parallel to the defect
ii. Flap undermined and advanced
iii. Useful for longitudinal defects of extremities
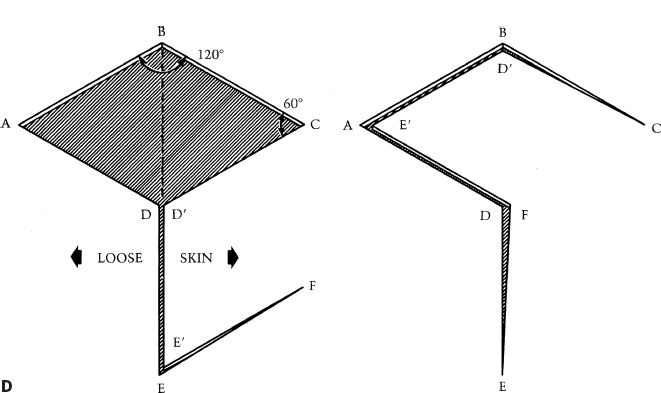
Figure 4-3. A: Single pedicled advancement flap with Bürow’s triangles. B: V–Y advancement flap. C: Rotation advancement flap. D: Rhomboid flap.
c. Island advancement flap
i. Pedicle of island flap not connected with surrounding skin, supplied only by connection with subcutaneous tissue (or artery/vein)
ii. Transferred by advancement or pivot (see below)
iii. Geometry of cutaneous portion depends on the defect
d. V–Y advancement flap (Fig. 4-3B)
i. Can be designed as advancement (where skin at base is not divided) or island flap (where skin at base of flap is divided)
ii. Flap raised in V shape and advanced to fill the defect
iii. Closed in Y to close the donor defect
iv. Flap may be modified to a Y–V variation
v. Useful on face and fingertips to fill defects (island design) or can be used to release contracture (advancement design)
2. Pivotal flaps: Moves around fixed point at base of pedicle
a. Rotation flap: Pivotal flap with curvilinear configuration (Fig. 4-3C)
i. Flap raised in semicircle immediately adjacent to the defect.
ii. Height of the defect = ½ to 1 times radius of flap curvature
iii. Standing cutaneous deformity may occur at base (depending on shape of the defect), requiring removal.
iv. Back cut at base of flap shifts pivot point to reduce tension of closure
v. Bürow’s triangle at secondary defect margin may help to facilitate closure. (corrects length discrepancy between flap margin and surrounding skin edge)
vi. Flap design with length of incision ˜4 times width of the defect typically does not require Bürow’s triangle to equalize length.
vii. Also uses some advancement or stretch of flap to correct length discrepancy between lengths of closure of flap margin and skin edge (often called rotation advancement flap).
viii. Undermining base of flap and pivot point facilitates advancement and limits standing cutaneous deformity.
ix. Flap should be inferiorly based on face to facilitate lymphatic and venous drainage.
x. Useful for scalp defects and sacral pressure sores (among other defects)
b. Transposition flap: Pivotal flap with linear configuration
i. Rhomboid (Limberg) flap (Fig. 4-3D)
a) Defect made into rhombic shape (equilateral parallelogram) with 60 and 120 degree angles.
b) First side of flap is a short diagonal of rhombus extended at an equal length.
c) Second side of flap is a line parallel to and same length as nearest limb of flap.
d) Four flaps can be designed around sides of the defect, flap design chosen with best skin mobility and scar placement.
ii. Dufourmentel flap
a) Variation of rhomboid flap
b) Used for rhomboid-like defects with angles other than classic 60 and 120 degrees.
c) First side of flap is same length of defect, drawn at angle halfway between a line extending from a short diagonal and a line extending from the side of the defect.
d) Second side of flap is parallel to long diagonal of the defect same length as the defect.
iii. Bilobed flap
a) Transposition of two flaps with common base
b) *Two flaps raised 45 to 50 degrees apart next to the defect (total arc of movement should be limited to 90 to 100 degrees)
c) First flap fills the primary defect, second flap fills donor site of first flap, and second flap donor site closed primarily.
d) Useful for nasal defects where site of the defect (nasal tip) does not have laxity for primary closure, but donor site of second flap (nasal dorsum) has sufficient laxity for closure.
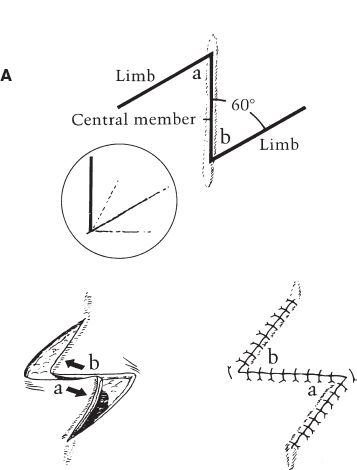
iv. Z-plasty (Fig. 4-4A)
a) Transposition of two adjacent and opposing triangular flaps
b) Used to lengthen scar contractures, change scar direction, break up scar, release epicanthal folds or constricting bands.
c) Moves lateral tissue inward from both directions in order to increase tissue length longitudinally (Table 4-1) at the expense of width.
d) Classic design: Central segment with limbs oriented at 60 degrees (although can be 30 to 90 degrees); three lines are equal in length; central segment oriented along the direction of the desired lengthening (e.g., in the same direction as scar to be released).
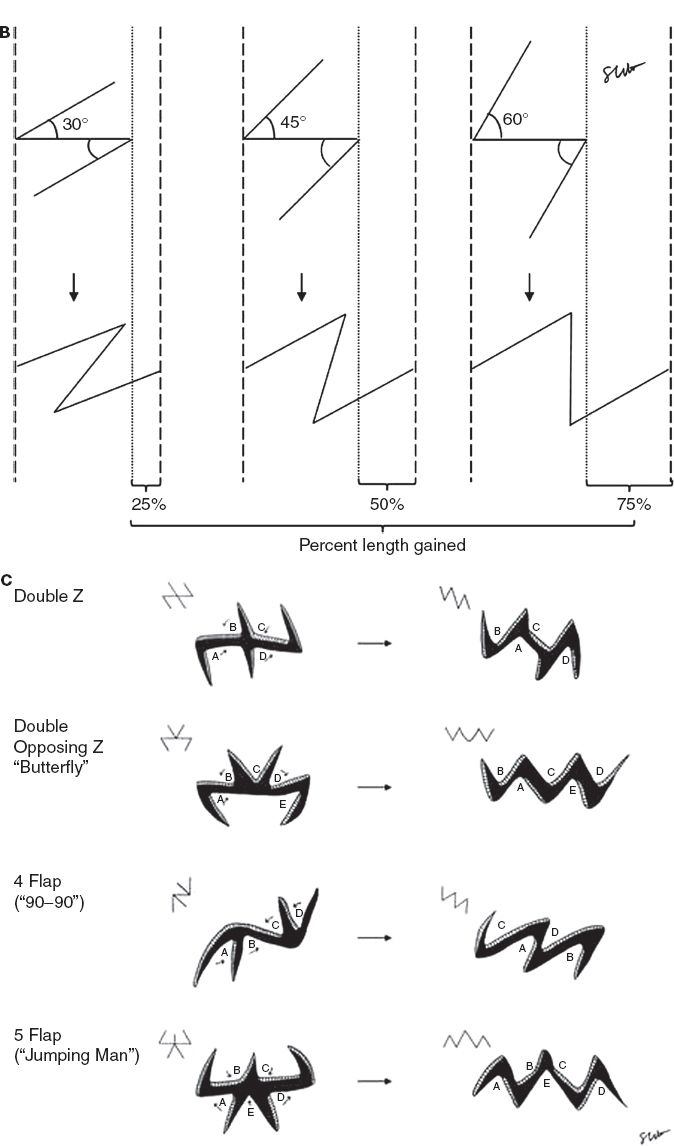
Figure 4-4. A: The standard Z-plasty. B: Percent length gained with the Z-plasty. C: Multiple Z-plasties.
e) Increasing angle of limbs increases percent gain in length along the central limb (Fig. 4-3B).
f) Multiple Z-plasties can be designed in series (Fig. 4-4C)
v. Interpolation flap
a) Skin paddle transposed into the nearby defect over or under a skin bridge.
b) Base of flap is not contiguous with the defect in initial procedure.
c) Flap is divided from base in secondary procedure and inset fully into the defect.
vi. Island transposition flap
a) Single-stage version of interpolation flap
b) Transferred by pivoting or advancement (see above)
vii. Other design configurations
a) Rectangular-shaped transposition flap
b) Parabola-shaped transposition flap
c) Triangular-shaped note flap
1) Closure of small (<2 cm) circular defect with triangular-shaped transposition flap
2) Tangent is drawn from any side of the circular defect 1.5 times length of diameter of the defect (parallel to relaxed skin tension lines)
3) Second side of flap same length as defect, drawn at 50 or 60 degree angle to first incision
4) Distal tip of flap is trimmed
3. Hinge flaps
a. Flap elevated in subcutaneous plane
b. Pedicle base is on one border of the defect
c. Flap is turned over into the defect like a book
d. Flap elevated with or without skin
i. Design with skin used in full-thickness defects that require internal lining (nasal reconstruction)
ii. Designs without skin require skin graft or second flap coverage
IV. FASCIAL FLAPS
A. Skin elevated with underlying deep fascia (fasciocutaneous flap) or fascia is elevated alone (fascial flap).
B. Indications
1. Need for thin, pliable coverage when bulk of muscle flap not desired.
2. Cases where secondary procedures anticipated under flap; fascial flaps easier to elevate during reoperative procedures.
3. Better gliding surface for coverage of exposed tendons.
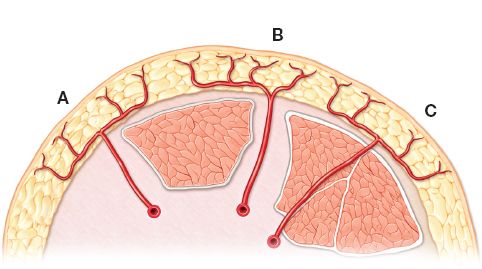
C. Blood supply/Mathes and Nahai classification (Fig. 4-5)
1. Type A: Direct cutaneous perforator; pedicle courses beneath deep fascia on its way to skin (e.g., temporoparietal fascia flap, groin flap).
2. Type B: Septocutaneous perforator; pedicle travels within intermuscular septa (e.g., radial forearm flap)
3. Type C: Musculocutaneous perforator; pedicle based on musculocutaneous perforators that travel through muscle to supply deep fascia and overlying skin (e.g., anterolateral thigh flap)
Figure 4-5. Mathes and Nahai classification of fasciocutaneous flaps. Type A: direct cutaneous perforator; Type B: septocutaneous perforator; Type C: musculocutaneous perforator.
D. Flap design
1. Flaps include deep fascia, incorporating rich vascular fascial plexus that reach the skin via direct or indirect perforators.
2. Type A and B pedicles are relatively constant in location; Type C pedicles have more variability in location
3. Pedicle can be lengthened by tracing perforators back to source vessel.
4. Fascia-only flaps advantageous because donor site can be closed primarily; fasciocutaneous flaps may or may not require skin graft to close donor site.
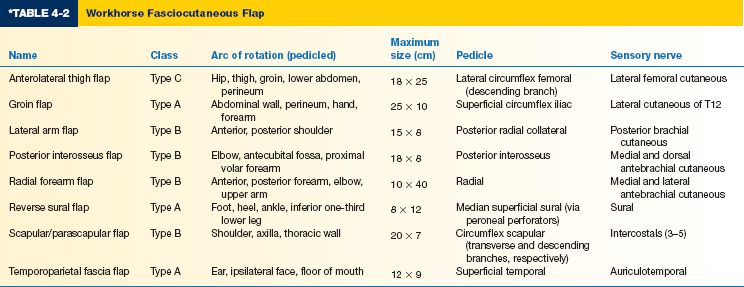
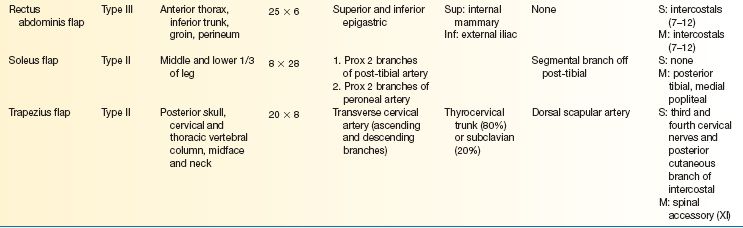
E. Workhorse fasciocutaneous flaps (Table 4-2)
V. MUSCLE FLAPS
A. Muscle can be transferred alone (muscle flap) or with overlying skin (musculocutaneous flap)
B. Indications
1. Muscle flaps are useful when a bulkier reconstruction is needed.
2. Eradication of dead space
3. Need for tissue with robust blood supply due to risk of infection or poor perfusion.
4. Restoration of motor function (functional muscle transfer)
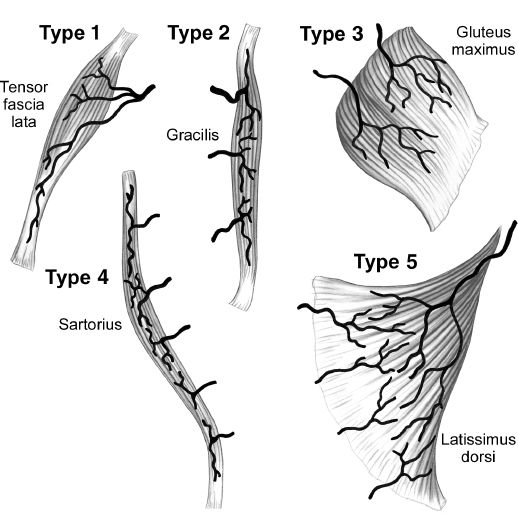
C. Blood supply/Mathes and Nahai classification (Fig. 4-6)
1. Type I: Single vascular pedicle (e.g., gastrocnemius, tensor fascia lata)
2. Type II: Single dominant pedicle and one or more minor pedicles; flap cannot survive on minor pedicles alone; most common type of muscle in body (e.g., soleus, gracilis, rectus femoris, biceps femoris)
3. Type III: Two dominant pedicles; flap can survive on either pedicle alone (e.g., rectus abdominis, gluteus maximus)
4. Type IV: Segmental pedicles; multiple pedicles enter along course of muscle, each supplies a portion of the flap; least reliable type (e.g., sartorius, tibialis anterior)
5. Type V: One dominant pedicle and secondary segmental pedicles; flap can survive on segmental pedicles alone (e.g., latissimus dorsi, pectoralis major)
D. Flap design
1. Skin island is designed to include skin perforators arising from the source artery.
2. Musculocutaneous perforators typically located near entry of dominant pedicle into hilum of the muscle.
3. All or part of muscle can be used as a flap.
Figure 4-6. Mathes and Nahai classification of musculocutaneous flaps. (From Berger RA, Weiss AC, eds. Hand Surgery. Philadelphia, PA: Lippincott Williams & Wilkins; 2004.)
4. May also include bone, motor nerve, or sensory nerve in transfer (depending on donor muscle).
5. Functional muscle is sacrificed, thus donor morbidity must be considered when selecting flap.
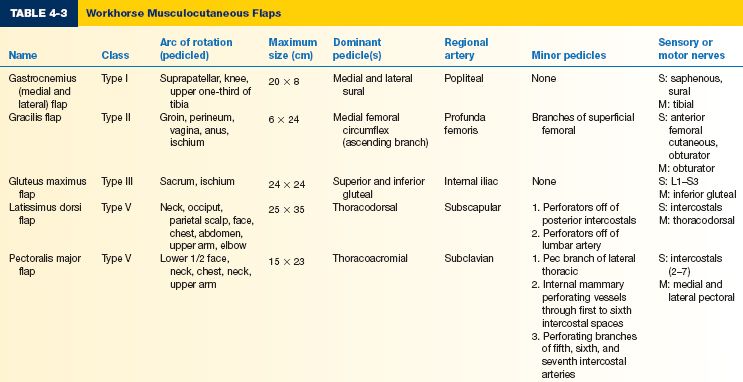
E. Workhorse musculocutaneous flaps (Table 4-3)
VI. FLAP MODIFICATIONS
A. Flap delay
1. Staged technique to augment flap circulation and improve flap survival.
2. Flap is partially elevated and entirely elevated, or selected pedicles are divided into one or more procedures; flap is brought back to in situ position in staged procedure before definitive flap elevation and transfer.
3. Allows harvest of larger flap because areas farthest from blood supply (random-supply component) have improved perfusion following delay.
4. Time between delay and definitive procedure is usually 2 weeks.
5. Delay used with flap locations or designs at high risk for compromised blood supply.
6. Physiology of improved perfusion with delay
a. Decrease in sympathetic tone from transection of sympathetic fibers.
b. *Dilation of previously closed choke vessels increases the area of tissue supplied by the dominant pedicle.
c. Relative tissue ischemia stimulates angiogenesis, increasing flap vascularity before transfer.
B. Crane principle
1. Pedicled flap used to lift, transport, and deposit subcutaneous tissue to recipient bed.
2. Flap is raised and transferred to recipient bed.
3. After 10 to 21 days, new blood vessels have grown in the recipient bed which will support a skin graft. In the next stage, the top layer (superficial one-half to three-fourths) is raised and returned to original donor site.
4. Viable subcutaneous tissue is left behind and can be covered with skin graft.
5. Provides coverage to local or regional area without significant donor site morbidity.
C. Flap prefabrication
1. Introduction of new vascular pedicle into tissue
2. Indication: When desired donor tissue has required qualities but does not have reliable axial blood supply.
3. Two-stage process
a. Stage 1: Transfer of a new vascular pedicle into an area of tissue that will be used to reconstruct defect (pedicle wrapped in Gore-Tex or silicone sheet to prevent scarring around pedicle).
b. Stage 2: The flap, based on new vasculature, can be raised after approximately 6 to 8 weeks (transferred as pedicle flap or free tissue transfer).
4. Rarely used because of the availability of many alternative flap options.
5. Can be performed with tissue expansion; introduced pedicle is placed beneath donor tissue and above tissue expander; Doppler flow is monitored during expansion.
6. Flap delay helps to hasten neovascularization.
7. Venous congestion is a common complication (may be lessened with flap delay).
D. Prelamination
1. Introduction of additional tissue layers into flap prior to transfer to create multilayer composite flap; allows tissue to have time to mature before transfer.
2. Indication: Allows custom-made flaps for specialized areas of the body with 3D structure (e.g., central face, penis).
3. Two-stage process
a. Stage 1: Modify donor flap by introducing additional tissue layer into vascularized tissue before transfer to recipient site (e.g., introduce cartilage and/or skin grafts to forehead flap before transfer to the defect).
b. Stage 2: Raise flap en bloc as composite flap and transfer to recipient site after approximately 2 to 4 weeks (shorter maturation time than prefabrication because vascular supply is not altered).
E. Supercharging
1. Enhancing blood supply of a pedicled flap by performing microvascular anastomosis to a secondary pedicle of the flap.
2. Example: Transverse rectus abdominis musculocutaneous flap with classic superior epigastric artery pedicle that also has deep inferior epigastric artery anastomosed to vessels in the axilla, neck, or chest to enhance blood supply.
F. Free tissue transfer
G. Perforator flaps: Perforating vessel(s) dissected down to deeper vessels, leaving intervening tissue intact and not included in flap to produce thinner flaps and reduce donor site morbidity.
H. Composite flaps
1. Angiosome principle provides basis for transfer of composite flaps that contain combinations of multiple tissue types (e.g., skin, muscle, bone, nerve, and/ or tendon).
2. Tissues supplied by single source artery can be transferred together.
3. Useful when reconstruction of multiple tissue components is needed.
4. Vascularized bone flaps
a. Blood supply classification (Serafin)
i. Direct (endosteal) circulation
ii. Indirect (periosteal) circulation
b. Commonly transferred bones and vascular pedicle
i. Radius: Radial artery
ii. Fibula: Peroneal artery
iii. Scapula: Circumflex scapular or thoracodorsal artery
iv. Iliac crest: Deep circumflex iliac artery
c. Toe transfer
i. Great toe: First dorsal metatarsal artery
ii. Second toe: First dorsal metatarsal artery
5. Innervated flaps
a. Motor nerve and/or sensory nerves are preserved or coapted to appropriate nerve near the recipient site.
b. Common functional muscle flap transfers and motor nerve
i. Gracilis with obturator nerve
ii. Latissimus with thoracodorsal nerve
iii. Serratus with long thoracic nerve
iv. Pectoralis minor with medial and lateral pectoral nerves
c. Common sensory flaps and sensory nerves
i. Lateral arm flap with posterior brachial cutaneous nerve
ii. Radial forearm flap with medial and/or lateral antebrachial cutaneous nerves
iii. Dorsalis pedis flap with deep peroneal nerve and/or superficial peroneal nerve
I. Chimeric versus conjoined (Siamese) flap
1. Chimeric flap: Has multiple territories, each with independent vascular supply (perforators or named branches), but territories are NOT connected except by connection to common source vessel.
2. Conjoined flap: Has multiple territories, each with independent vascular supply, but territories remain connected.
J. Tissue expansion
VII. POSTOPERATIVE MANAGEMENT
A. Flap monitoring
1. Evidence of arterial or venous insufficiency in immediate post-op period requires immediate exploration
2. *Clinical evaluation: Gold standard method of flap assessment
a. Temperature: Should be body temperature
b. Color: Should be pink
c. Capillary refill: Should be approximately 2 seconds
d. Bleeding: Upon introduction of fine-gauge needle, bright-red bleeding should be present
e. Firmness: Should be soft, with some appreciable turgor
3. Additional methods of flap monitoring
a. Doppler (implanted or external)
b. Fluorescein dye
c. Pulse oximetry, pH, or temperature sensors
4. Signs of arterial insufficiency
a. Cool temperature
b. White color
c. Slow capillary refill >2 seconds
d. Slow or absent pinpoint bleeding
e. Low turgor
5. Signs of venous insufficiency
a. Increased temperature
b. Blue to purple color
c. Brisk capillary refill <2 seconds
d. Brisk pinpoint bleeding, dark in color
e. Increased turgor, tense, swollen
f. If congested, unwrap, release sutures and consider leech therapy (patient must be on quinolone or third generation cephalosporin against Aeromonas)
B. Risk factors for flap vascular compromise
1. Tight dressings and/or splints
2. Tight sutures
3. Patient position or motion that puts pressure on flap
4. Hematoma (increases tissue pressure and interferes with perfusion)
5. Kinking of flap, pedicle, or both (may be influenced by flap design, pedicle length, interpositional vein graft length)
6. Cool ambient room temperature
7. Poor surgical technique
8. Systemic patient factors: Use of vasoconstrictive pharmaceutical agents (vasopressors, nicotine, caffeine, etc.), hypovolemia, anemia, inadequate blood pressure
QUESTIONS YOU WILL BE ASKED
1. What is the most common (Mathes and Nahai) type of muscle flap in the body?
Type II
2. What is the physiology behind improved perfusion after flap delay?
Flap perfusion is increased through (1) decrease in sympathetic tone from transection of sympathetic fibers, (2) dilation of previously closed choke vessels which increases the area of tissue supplied by dominant pedicle, and (3) relative tissue ischemia stimulates angiogenesis, increasing flap vascularity before transfer.
3. What is the theoretical gain in length along the central limb of a Z-plasty with 60 degree angle?
75% gain in length
4. What is the pedicle to your flap?
5. What is difference bertween a type II and type V flap?
Type II flap has secondary pedicle that cannot support the flap alone. Type V flap can be transfered on the secondary pedicles which are segmental.
THINGS TO DRAW
1. Z-plasty before and after
2. Cross section through lower extremity
Recommended Readings
Ghali S, Butler PE, Tepper OM, Gurtner GC. Vascular delay revisited. Plast Reconstr Surg. 2007;119(6):1735–1744. PMID: 17440348.
Hallock GG, Morris SF. Skin grafts and local flaps. Plast Reconstr Surg. 2011;127(1):5e–22e. PMID: 21200192.
Taylor GI. The angiosomes of the body and their supply to perforator flaps. Clin Plast Surg. 2003;30(3):331–342, v. PMID: 12916590.
Taylor GI, Palmer JH. The vascular territories (angiosomes) of the body: experimental study and clinical applications. Br J Plast Surg. 1987;40(2):113–141. PMID: 3567445.
< div class='tao-gold-member'>