Fillers in Ethnic Skin
Frederick Beddingfield III
Jenny Kim
There has been rapid growth in the number of surgical and nonsurgical cosmetic procedures in darker racial ethnic groups, which accounted for approximately 20% of all cosmetic procedures in 2005. This group is comprised of Hispanics 9%, African Americans 6%, Asians 4%, and all other non-Caucasians 1.3%.1 Non-Caucasians are slightly less likely to obtain cosmetic procedures versus their Caucasian counterparts; nonetheless, ethnic minorities account for a significant population interested in cosmetic procedures and will likely account for a higher percentage of such cosmetic procedures in the future as this segment of the population is growing rapidly.
Dermal fillers are the third most common nonsurgical cosmetic procedure performed and are used to correct a range of skin defects, from fine lines to deeper nasolabial folds, lip augmentation, and volume correction of the cheeks. Collagen fillers were historically the most commonly used dermal fillers and have been used with great success for more than 20 years. However, hyaluronic acid fillers, because of their longer duration and excellent safety record, have become the most common filler injectables and the gold standard against which others are compared. In the United States in 2005, hyaluronic acid dermal filler injections were the third most common nonsurgical cosmetic procedure, behind botulinum toxin injections and laser hair removal.1 The use of hyaluronic acid filler injections saw a 35% growth rate from 2004 despite a 4% decline in nonsurgical cosmetic procedures overall. In 2005, patients had 1.2 million hyaluronic acid injections versus 221,000 collagen injections, 91,000 autologous fat injections, 40,000 calcium hydroxylapatite filler injections, and 35,000 L-poly lactic acid filler injections.1 This chapter will describe the characteristics of various fillers presently being used and discuss important factors to consider when fillers are used in patients with darker-skinned ethnic groups.
Hyaluronic Acid Fillers
The development of hyaluronic acid (HA) fillers has been an important advance because they have significant advantages over many collagen fillers—particularly longer duration. HA is a glycosaminoglycan consisting of D-glucuronic acid and N-acetyl-D-glucosamine disaccharide units and is a component of the extracellular matrix of skin connective tissue found in the epidermis and dermis. Additionally, it is found in vitreous humor, umbilical cord, synovial fluid, and the capsule of certain microorganisms.
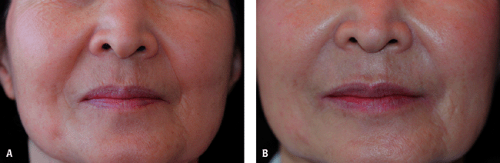
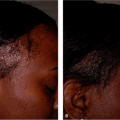
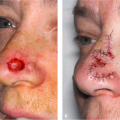
Various HA products are now available, and the most widely used HA fillers around the world are the Restylane, Hylaform, and Juvéderm families of products (Table 23-1). Restylane and Juvéderm products are all derived from streptococcal bacteria, and Hylaform products are derived from rooster combs. Unmodified nonanimal HA produced by bacterial fermentation process is identical to HA in humans and it is not a protein. It offers excellent biocompatibility and, there is no need for skin testing.2 Hypersensitivity reactions are rare and not expected. However, hypersensitivity reactions are possible as a result of manufacturing-related non-HA components of fillers. Thus, Hylaform products are contraindicated in patients who are hypersensitive to avian proteins because they are derived from rooster combs. Additionally, unlike collagen, there is no need for overcorrection during the injection procedure. Thus, HA fillers are particularly useful for patients who may react to collagen fillers or who desire immediate and predictable clinical improvement without the need to wait several weeks for the results of skin tests (Fig. 23-1A,B).
Naturally occurring HA contributes to adhesion, elasticity, and viscosity of extracellular substances. Comparative studies suggest HA fillers may be more durable than collagen fillers.3,4 Furthermore, HA is highly hydrophilic and is able to hold more water than any other natural substance. Once injected, it attracts water and has a hydrating effect on the skin. Although HA alone is degraded in the body within only a few hours, it can be stabilized by cross-linking so that its effects are far more prolonged. The longer duration of HA relative to collagen appears to be the primary reason why dermal filler use has increased so much in the United States since the approval of Restylane. Most HA fillers product lines are composed of two or three related formulations to enable a wide range of clinical
needs to be met. These subtypes commonly differ in rate of cross-linking, size, and formulation of HA strands or particles, and HA concentration. Typically one formulation is intended for the treatment of fine lines, a middle formulation is intended for treating medium-depth lines, and one formulation is intended for resolving deeper defects or replacing volume. Statistics from the American Society for Aesthetic Plastic Surgery indicate that the use of HA fillers in the United States grew almost 700% between 2003 and 2004.1
needs to be met. These subtypes commonly differ in rate of cross-linking, size, and formulation of HA strands or particles, and HA concentration. Typically one formulation is intended for the treatment of fine lines, a middle formulation is intended for treating medium-depth lines, and one formulation is intended for resolving deeper defects or replacing volume. Statistics from the American Society for Aesthetic Plastic Surgery indicate that the use of HA fillers in the United States grew almost 700% between 2003 and 2004.1
Table 23-1 Hyaluronic acid fillers | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Collagen Fillers
Bovine collagen
Bovine-derived collagen fillers were first approved for soft-tissue augmentation in the 1980s in the United States.These fillers set the standard in filler materials for smoothing facial lines, wrinkles, and scars and in providing lip border definition until HA products became available recently. More than a million treatments have been performed with bovine collagen, and the most widely used products include Zyderm I, Zyderm II, and Zyplast (Table 23-2). The content of collagen concentration varies in these products. For example, Zyderm I contains 95% to 98% type I collagen with some type III collagen. It has 3.5% bovine collagen by weight. Zyderm II is similar to Zyderm I except that it contains 6.5% collagen by weight. Neither Zyderm I nor II are cross-linked. Zyplast has 3.5% bovine collagen cross-linked by glutaraldehyde to form a latticework and is considered less immunogenic and more resistant to degradation than Zyderm I and Zyderm II. All three products contain 0.3% lidocaine and therefore are contraindicated in persons who have lidocaine allergy. Furthermore, these products are contraindicated in patients with hypersensitivity to bovine collagen. Approximately 5% of patients may experience hypersensitivity to injectable bovine collagen and so skin testing is required before treatment. At least two skin tests should be performed at least 2 weeks apart, with the last test at least 2 weeks before treatment for new patients or anyone who has not received the same product within 2 years. A positive test result is defined as erythema, induration, tenderness, or swelling that persists for more than 6 hours after implantation. If any hypersensitivity occurs, it is usually within 1 to 2 weeks of treatment and manifests as erythema and induration, with or without pruritus in the area treated. Treatment of hypersensitivity may include topical immunomodulatory calcineurin inhibitors, topical steroids, intralesional steroids, systemic steroids, and systemic cyclosporine. Observation alone may be enough in mild cases. Contraindications to injectable bovine collagen include a history of an anaphylactic event of any cause, previous sensitivity to bovine collagen, lidocaine sensitivity, pregnancy, and active infection at the treatment site. Although no formal testing has been completed, patients undergoing hormonal fluctuation (e.g., during pregnancy or the menopause) may have an increased risk for hypersensitivity.
Bovine collagen treatments reportedly last between 3 and 18 months, with an average duration of 2 to 6 months, depending on location. In the authors opinion, bovine collagen lasts approximately 2 to 3 months in the nasolabial folds and significantly less in the lips. Zyderm I is injected into papillary dermis to correct superficial facial rhytides. Zyderm II is injected slightly deeper to treat moderate rhytides and scars. Zyplast, injected into the deeper dermis for treatment of moderate to severe rhytides and scars, can be expected to last longer than Zyderm I or II. However, Zyplast is contraindicated in the glabellar area due to reports of skin necrosis after injection into this area.
Table 23-2 Collagen fillers | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|