Vulvar skin type
Epithelial characteristics
Associated diseases
Labia majora and outer minora
Keratinized stratified squamous epithelium
Psoriasis
Eccrine, apocrine, and sebaceous glands
Lichen sclerosus
Allergic and irritant dermatitis
Atopic eczema
Inner labia minora
Nonkeratinized stratified squamous epithelium
Lichen planus
No adnexal structures
Vaginal vestibule
Mucosa
Lichen planus
Vulvovaginal candidiasis
Atrophic vulvovaginitis
No adnexal structures
It appears that female-specific temporal shifts in the expression of hormones and receptors modulate cyclical changes in the skin’s basic composition. For example, it has been noted that keratinocytes have estrogen receptors that respond to rising and falling levels of estrogen. Through these receptors, estrogen can directly generate changes in skin hydration, collagen content, and in the concentration of glycosaminoglycans that form the skin barrier and, as a result, affect the sensation of itch [1]. Downstream effects include changes in vulvovaginal pH and varying microflora compositions [1]. Alterations in pH may be an important factor in the aggravation of itch since increasing pH is known to activate the PAR2 receptor, a well-known itch mediator. Given the extensive alteration of hormones throughout a female lifetime, there exists a diversity of skin pathologies and phenotypes that tend to fall into hormonal-dependent groupings (prepubertal, reproductive age, postmenopausal).
These conditions are known to cause significant discomfort, significantly impacting the quality of women’s lives. However, little effort has been placed on studying female-specific itch to date. This is particularly true in the case of vulvar dermatoses, which are often under-recognized and undertreated. Herein, we will examine the causes, manifestations, and management options of vulvar itch in women from childhood to postmenopause (Fig. 10.1).
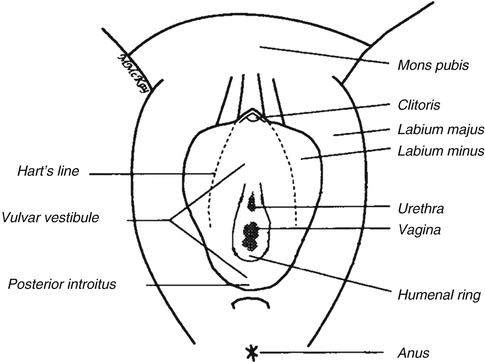

Fig. 10.1
Vulvar anatomy showing Hart’s line, the demarcation between keratinized (lateral to Hart’s line) and nonkeratinized epithelium (Figure courtesy of Marilynne McKay, MD)
10.2 Prepubertal Vulvar Pruritus
From birth to menarche, the vulvar and vaginal epithelium are characterized by low estrogen levels, a high vulvovaginal pH, and a lack of genital lactobacillus colonization. The main pruritic dermatoses in this age range are atopic and irritant dermatitis, psoriasis, and lichen sclerosus. Of note, streptococcal infection of the vulva occurs exclusively in prepubertal girls. Finally, poor hygiene, foreign bodies, and sexual abuse should also be considered as causes of vulvar pruritus in this age group.
10.2.1 Atopic and Irritant Dermatitis (Fig. 10.2)
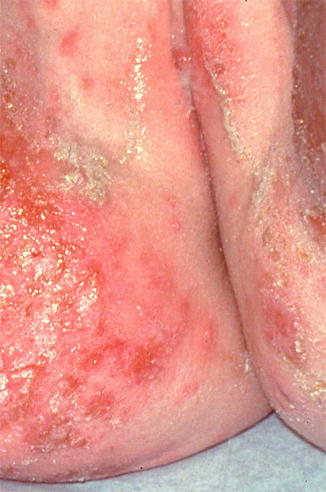
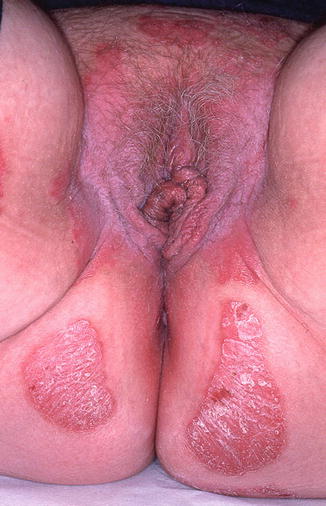
Fig. 10.2
Secondarily infected dermatitis in an atopic child with intense vulvar itching. Staphylococcus and streptococcus were both present (Photograph courtesy of Marilynne McKay, MD)
Atopic and irritant dermatitis are the most common causes of prepubertal vulvar itch, and they often occur together [2]. These conditions manifest as fluctuating, poorly defined erythematous patches and plaques involving the vulvar area that are exacerbated by excessive washing and overuse of antifungal creams [3]. The labia majora may have scale and slight rugosity, while the labia minora can present with desquamation and redness [3]. The pruritus is often so intense that scratching is difficult to control. As such, disturbances in sleep and parental embarrassment are not uncommon.
While superinfection by Staphylococcus aureus may occur, it rarely yields positive cultures [3]. Management of atopic and irritant dermatitis focuses on the reduction of irritant exposures (commonly urine, feces, soaps, and bubble baths), as well as application of low-dose topical steroids [2]. Secondary skin infections can be treated with topical antimicrobials such as mupirocin 2 % ointment, although more severe cases may require oral antibiotics.
10.2.2 Psoriasis
Vulvar psoriasis more commonly affects children than adults [2]. These lesions first present as a persistent diaper rash in babies. As children age, the lesions become pruritic, well demarcated, symmetric red plaques without scale in the vulvar and perianal regions [3]. When psoriasis is limited to the vulva, diagnosis may be difficult. In such cases, the presence of other manifestations of psoriasis such as nail pitting, history of cradle cap, and scalp/postauricular rashes can help confirm the etiology [3]. Vulvar psoriasis is managed with high-potency topical steroids or topical tacrolimus.
10.2.3 Lichen Sclerosus (Fig. 10.3)
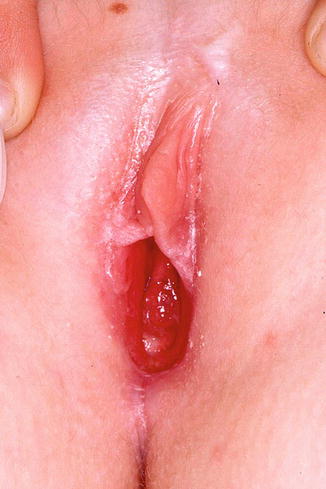
Fig. 10.3
Lichen sclerosus in a 7-year old. Note pale appearance of the inner labia majora with early resorption of the labia minora. Early involvement of the clitoral hood is typical and the perineal body shows early atrophic changes. The vagina is easily visualized due to loss of vulvar architecture (Figure courtesy of Marilynne McKay, MD)
Lichen sclerosus (LS) affects approximately 1 in 900 girls in the United States, with 7–15 % of all cases found in the prepubertal age group [4]. This chronic, autoimmune, mucocutaneous inflammatory dermatosis of unknown etiology classically presents as a white plaque with secondary atrophy and subcutaneous hemorrhage of the vulvar and perianal skin [2] in a figure of eight configuration. Extragenital lesions include pale “confetti” spots, which can occasionally be seen on the inner wrists or elsewhere on the body; these macules are often asymptomatic. The vulvar rash is characterized by severe pruritus, which may lead to subsequent soreness, dysuria, and chronic constipation [3]. The presence of petechiae and purpura can trigger inappropriate investigations into sexual abuse in this age group [2]. LS typically first presents between the ages of 4 and 5 years old, but diagnosis often lags for at least 1 year after onset of symptoms [4]. Complications include loss of genital architecture secondary to scarring and subsequent effacement of the labia minora and clitoris [5]. While some pediatric LS resolves with puberty, other cases may silently progress into adulthood. Long-standing LS is associated with a less than 5 % chance of squamous cell carcinoma later in life [6–8]. Treatment of LS includes high-potency topical steroids and topical calcineurin inhibitors with frequent follow-up [5].
10.2.4 Infectious Vulvovaginitis
Infectious vulvovaginitis in the female prepubertal age group typically occurs secondary to group A beta-hemolytic streptococcal infection [9]. Streptococcal bacterial infections of the vulva may present in acute or subacute forms. In the more severe, acute form, patients present with erythematous, painful, edematous plaques with discharge. Alternatively, they may present with subacute inflammation, manifested as pruritic erythematous patches and plaques in the vulvar and perianal regions. Streptococcal infections are diagnosed via culture and sensitivity of vaginal swabs. Treatment includes oral antibiotics such as penicillin, amoxicillin, or cephalexin (if penicillin allergic). Although streptococcus accounts for most cases of infectious vulvovaginitis in these prepubertal girls, more rare infections include staphylococcus, haemophilus, and shigella [2]. Beyond bacterial infections, pinworm is a common etiology of vulvar and perianal pruritus and may be associated with an eczematous rash. Mebendazole is the treatment of choice for pinworm [3]. Although tinea infections can occasionally be seen in girls, vulvovaginal candidiasis does not occur before menarche in immunocompetent patients.
10.3 Reproductive-Age Vulvar Pruritus
With the onset of menstruation, baseline estrogen levels rise and cyclic hormonal changes are triggered, creating a new cutaneous environment. At puberty, estrogen begins to act on maturing keratinocytes, causing vulvovaginal pH to decrease from an average of 7 in prepubertal girls to an average of 4 in adult females [10]. During this hormonal transition, the vulvar epithelium becomes rich in glycogen, and lactobacilli begin to colonize the vulvovaginal area. After menarche, monthly variations in vaginal physiology and pH significantly alter the cutaneous environment. In the first 2 weeks of the menstrual cycle, estrogen levels rise, and vulvovaginal epithelial cells proliferate. In the second 2 weeks, progesterone reigns as the primary hormonal player, and as a result, these keratinocytes desquamate [11]. Cyclical hormonal changes also modulate the bacterial flora composition of the vulvovaginal region. Finally, hormonal pH changes associated with the menstrual cycle have a direct relationship with itch stimulation, as increasing pH is known to activate a well-known itch mediator, the PAR2 receptor [12].
Common causes of vulvar pruritus in reproductive-age, nonpregnant women include vulvovaginal candidiasis, allergic and irritant dermatitis, lichen simplex chronicus, psoriasis, and to a lesser extent lichen sclerosus. Other, less common, causes are lichen planus, vaginal infections, herpes vulvovaginitis, and seborrheic dermatitis.
10.3.1 Vulvovaginal Candidiasis
Most reproductive-age women experience at least one episode of vulvovaginal candidiasis (VVC) in their lifetime, and approximately half of these women endure multiple episodes [13]. Estrogen mediates the colonization of the vulvovaginal region with yeast. Because of this hormonal control, vulvovaginal candidiasis occurs almost exclusively in the reproductive years. Since estrogen levels are highest in the premenstrual period, candidal infections occur more commonly during the second half of the menstrual cycle [13]. Among women of different age groups, vulvovaginal candidal colonization has been estimated to have a prevalence of 11–22 % [14–16]. Certain conditions and medications may increase estrogen levels and lead to more frequent colonization as well as infections. These include pregnancy, antibiotic use, the use of hormonal birth control methods, hormone replacement therapy, and tamoxifen [2, 13–16]. Changes in immune regulation can also cause yeast infections, including diabetes, HIV, thyroid disease, lupus, corticosteroid use, and inheritance of a polymorphism associated with low production of mannose-binding lectin [13].
The typical presentation of VVC includes itching and burning of the vulva, with or without white discharge and vulvovaginal redness. Some patients also experience dysuria and dyspareunia. It should be noted that VVC is largely overdiagnosed in women with vulvar itch; self-diagnosis is poor, particularly in cases of recurrent vulvovaginal symptoms [13]. Diagnosis is usually made by wet prep to visualize fungal elements. However, when wet prep is indeterminate, culture or PCR may be used along with a normal vaginal pH to rule out bacterial vaginosis, atrophic vaginitis, and trichomoniasis [13]. Treatment involves topical or systemic antifungal azoles, with expected resolution of symptoms in 2–3 days [13]. Older proven therapies include topical treatment with Silvadine 1 %.
Recurrent VVC is defined as the occurrence of at least four episodes within 1 year or at least three episodes in 1 year not associated with antibiotic use [2]. These women are usually otherwise healthy and develop a hypersensitivity-like reaction to Candida. Recurrent vulvovaginal candidiasis (RVVC) may require a long-term treatment regimen of a weekly or biweekly suppressive azole antifungal [18].
Most VVC cases are associated with Candida albicans, although other species such as C. glabrata, C. tropicalis, and C. parapsilosis should be considered in relatively treatment-resistant cases. In such circumstances, fungal culture, rather than wet mount alone, is necessary for diagnosis [17].
10.3.2 Allergic and Irritant Contact Dermatitis (Fig. 10.4)
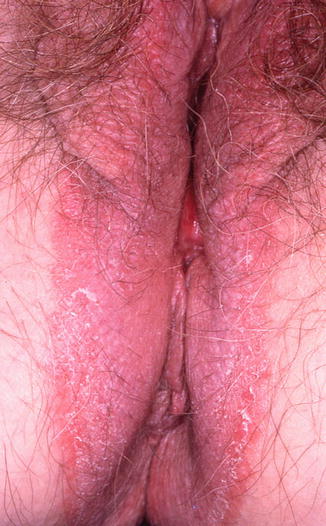
Fig. 10.4
Chronic contact dermatitis due to neomycin ointment. Note that the rash extends into the gluteal cleft and perineal area where the patient applied the medication (Figure courtesy of Marilynne McKay, MD)
Approximately 50 % of chronic vulvovaginal pruritus cases are due to allergic and irritant contact dermatitis [13]. These dermatoses present in a nonspecific fashion, with sudden or gradual onset of itching, burning, and erythema of the vulvovaginal region. The common offending agents in allergic and irritant dermatitis are displayed in Table 10.2.
Allergic contact dermatitis | Neomycin |
Clobetasol | |
Benzocaine | |
Lanolin | |
Dyes (clothing and black hair dye) | |
Thiuram (in rubber condoms) | |
Sanitary pads | |
Perfumes | |
Sodium metabisulfite (in topical antifungal creams) | |
Irritant contact dermatitis | Antifungal or menstrual-related topical creams and liquids |
Harsh soaps and antiseptics | |
Urine | |
Douches | |
Lubricants and spermicides | |
Tampons and sanitary pads | |
Synthetic underwear |
In acute irritant contact dermatitis, redness can be localized to the area of contact (if in solid or cream form) or may be diffuse (if in water soluble or liquid form). Subacute and chronic cases are characterized by erythema, swelling, and lichenification of the affected area. While vesicles and bullae occur with irritant dermatitis on other parts of the body; they are uncommon on the vulva [13]. New popular types of cosmetic hair removal may make the skin more sensitive to a host of irritants that were previously unknown.
Allergic contact dermatitis of the vulva has a similar presentation to irritant contact dermatitis, but may be delayed or intermittent in nature. Continuous exposure to an allergen triggers an itch-scratch cycle that leads to the development of the typical thickened plaques of lichen simplex chronicus. Medical history is the key to accurate diagnosis. While patch testing is not routinely performed, it can be helpful in certain cases. Allergic contact dermatoses are treated by removing the offending agent, avoiding overwashing, and applying topical steroids.
10.3.3 Lichen Simplex Chronicus and Neuropathic Itch (Fig. 10.5)
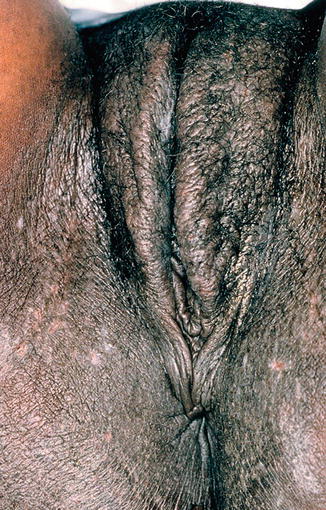
Fig. 10.5
Lichen simplex chronicus (LSC) due to chronic rubbing and scratching of pruritic skin. The surface shows thickening with increased skin markings and hairs have been broken due to trauma. Hyperpigmentation is a common finding in black patients with LSC (Figure courtesy of Marilynne McKay, MD)
Lichen simplex chronicus (LSC) occurs as a result of chronic rubbing and scratching of the skin. On the vulva, the skin becomes thickened, lichenified, and often hyperpigmented. Various etiologies of LSC exist; the condition can occur secondary to pruritic conditions such as LS or allergic contact dermatitis (ACD). Alternatively, it may be part of a systemic neuropathy or, in some cases, is a primary psychogenic process [13]. When LSC is suspected in the vulvar area, it is important to rule out neuropathic itch associated with sacral spinal compression: a lumbar X-ray may be helpful in identifying possible involvement of the dorsal root ganglia [19]. Other types of neuropathic itch include postherpetic neuralgia and diabetic neuropathy. To successfully treat neuropathic itch and LSC, patients must break the cycle of itching and scratching. This may be accomplished with behavior modification, anti-itch medications, anticonvulsants such as gabapentin and pregabalin [20], and topical steroids.
10.3.4 Psoriasis (Fig. 10.6)