I. FAT GRAFTING
A. Background
1. Eugene Hollander documented the first known use of fat in surgical enhancement in 1912 when he used this technique in patients presenting with lipoatrophy of the face.
2. Report of fat grafting that quoted poor outcomes in patients who had received autologous lipoaspirate transplants in the 1980s delayed acceptance of this technique.
3. Eventually, surgeons started using fat grafting to treat a variety of conditions, including radiation damage, vocal cord impairment, breast capsular contracture, chronic ulceration, and, ultimately, burn injuries.
4. In general, “fat grafting” may be used to describe three alternatives.
a. Composite grafts of fat that have been minimally modified/processed.
b. Modified and processed portions of fat that can be injected after processing has been completed.
c. Highly sorted adipose-derived mesenchymal stem cells (MSCs) organized by fluorescence-activated cell sorting.
5. Adipose tissue composition
a. Macroscopically, at least five different types of adipose tissue exist: bone marrow, brown, mammary, mechanical, and white. Each serves a distinct biological function.
b. Adipose tissue is composed mainly of fat cells organized into lobules
i. Mature adipocytes (90% volume)
ii. Stromal vascular fraction (SVF): preadipocytes, fibroblasts, vascular smooth muscle cells, endothelial cells, resident monocytes/macrophages, lymphocytes, and adipose-derived stem/stromal cells (ASCs).
c. Adipocytes account for approximately 20% of all cells within the subcutaneous tissue.
d. Biochemistry
i. Adipocytes contain two receptors for catecholamines, which regulate fat storage.
ii. β-1 receptors—located in areas of metabolically active fat such as upper body, face, and breast—respond to catecholamines by releasing lipase, splitting triglyercides into glycerol and fatty acids.
iii. α-2 receptors—located in diet-resistant areas such as the lateral thighs, buttocks, and abdomen—are antagonists of β-1 receptors and block lipolysis.
B. Indications
1. Tissue augmentation in any subcutaneous location with tissue atrophy. Most commonly used in the face: nasolabial folds, lips, malar region, and cheek.
2. Lipodystrophic syndromes and atrophic areas.
3. Breast reconstruction.
4. Breast augmentation.
5. Scar revision.
6. Hemifacial microsomia.
______________
*Denotes common in-service examination topics
1. Coleman technique
a. 3-mm incisions.
b. Blunt tip attached to 10-mL Luer-Lok syringe.
c. Cannula pushed through harvest site as surgeon uses manipulation to create gentle negative pressure by pulling back on plunger.
d. Plunger removed and syringe placed in centrifuge.
2. Harvest can be done by liposuction.
3. Different providers have different preferences and beliefs about the effect of amount of suction during harvest, length of canula, and size of collection tube but no studies have shown difference in adipocyte survival.
4. Donor site has not proven to affect outcome: Most commonly abdomen, buttocks, and thigh.
5. Superwet or tumescent techniques avoided to prevent trauma to graft during harvest. (Can use 1 mL of local anesthetic per mL of fat harvest).
6. Can inject tumescent fluid after harvest for hemostasis and pain control.
D. Processing
1. Coleman technique: Lipoaspirate is loaded into 10-mL syringes.
2. Adipose tissue centrifuged at 3,000 rpm or 1300G for 3 minutes (though different providers use different settings).
3. Centrifugation: Separates aspirate based on density.
a. Less dense components: Adipocytes with part of stromal vascular fraction.
b. More dense blood, lymphocytes, as well as part of the stromal vascular fraction.
4. Blood and tumescent fraction drained from the bottom layer and the oil is decanted and wicked with a cotton pledget for 3 minutes from the top layer.
E. Placement of fat graft
1. Smaller gauge used than harvesting.
2. Blunt tip allows less traumatic introduction (Coleman: 17G cannula with a 1-mL syringe, injecting tiny amounts with each pass).
3. Fat should be placed with multiple passes laying down single layer of fat and avoiding clumping.
4. Each injection should be a new tunnel creating multiple levels in a three- dimensional manner.
5. If clump injected, flatten with digital manipulation.
6. Usually placed just under the dermis.
7. Augmentation over mandible and malar region just over periosteum.
8. Possible methods to improve survival
a. Greater exposure of each adipocyte to vasculature.
b. Diffuse infiltration with multiple passes.
c. Small amount of placement per pass.
d. Large surface area of contact between fat and surrounding tissue.
e. Pure fat.
f. Once you feel large open space with each pass, probably it is good to stop.
9. Three zones of healing among the adipocytes located in a newly fat-grafted bed
a. Necrotic zone.
b. Regenerating zone.
c. Surviving zone: Both adipocytes and ASCs survive.
F. Specific clinical uses
1. Facial volume correction
a. Injection of 16G needle stab incisions on each side of oral commissure and placement into upper lip, perinasal area.
b. Inject in vermilion of lip to roll out vermilion and give patient more red lip.
c. Submalar area with 18G blunt with 0.05 cc per pass.
d. Can use pickle fork to folds.
e. Periorbital rejuvenation: Inject along inferior orbital rim in plane close to the bone.
f. Temporal rejuvenation: Fat placed subcutaneously in plane above the temporalis fascia.
a. Three weeks of expansion via Brava System.
b. Injection using 14G Coleman side hole needle with multiple sites of injection along inframammary fold.
c. Fat injected subcutaneously and not directly into the breast tissue.
3. Breast reconstruction
a. Postmastectomy or postlumpectomy deformity can be used to improve contour irregularities in tissue expander and autogenous breast reconstruction.
b. Tuberous breast deformity.
c. Poland’s syndrome.
a. Can harvest fat using in-line machine and liposuction.
b. Injection cannula 16G blunt.
c. Inject subdermal.
d. Often requires multiple stages for large deformities.
G. Graft survival and healing
1. Patients can have considerable postoperative edema due to multiple passes with injection.
2. In general, graft viability varies from as high as 70% to as low as 30%. Survival rate decreases with infection and trauma.
3. Overcorrection needed to optimize results and account for graft loss. Overcorrection by 50% recommended (although debated).
4. Final volume determined by
a. Interactions between multiple cell types, including ASCs, viable adipocytes, and necrotic adipocytes.
b. These cells stimulate maintenance of a set volume as determined by the original composite of tissue transferred into the wound bed.
5. For most patients included in studies examining the effect of fat grafting after thermal or radiation injury, an average of two treatments are needed.
6. Often the second fat transplantation occurs 3 months after the initial procedure.
H. Complications
1. *Glabellar injection can cause blindness.
2. Skin necrosis.
3. Fat resorption and necrosis.
4. Irregularities.
5. Unknown risk in breast cancer and head and neck patients.
II. ADIPOSE-DERIVED STEM CELLS
A. Can be isolated from adipose tissue harvested either by liposuction or by excision of tissue.
B. If harvested by liposuction, adipose tissue settles into two layers
1. Supernatant or processed lipoaspirate (PLA) layer: Consists of the suctioned adipocytes as well as their surrounding endothelium and stroma.
2. The bottom layer or liposuction aspirate fluid: Consists of injected saline, erythrocytes, and denser pieces of the PLA layer.
3. ASCs can be harvested from both layers; however, the yield of adherent ASCs is significantly higher in the adipocyte layer than in the liposuction aspirate fluid cells.
a. Terms for adipose-derived “stem cells”: ASCs, adipose-derived adult stem cells (ADAS), adipose-derived adult stromal cells, adipose-derived stromal cells (ADSCs), adipose stromal cells (ASCs), adipose MSCs (AdMSCs), lipoblast, pericyte, preadipocyte, and PLA cells.
b. International Fat Applied Technology Society reached a consensus: ASCs to describe plastic-adherent, multipotent cell population.
c. The exact location of ASCs is unknown: ASCs may exist within the perivascular tissue since they express similar cell surface antigens to pericytes.
d. Culturing of these cells eventually results in the appearance of a relatively homogeneous population of mesodermal or MSCs (usually after two to three passages) after nonadherent cells from SVF are washed away.
e. Beneficial impact of ASCs may be due to soluble factors produced by ASCs rather than their differentiation capability toward different mature lineages.
f. ASCs secrete hepatocyte growth factor (HGF), vascular endothelial growth factor (VEGF), transforming growth factor-β (TGF-β), insulin-like growth factor (IGF)-1, basic fibroblast growth factor (bFGF), granulocyte–macrophage colony-stimulating factor, tumor necrosis factor (TNF)-α, interleukin-6, interleukin-7, interleukin-8, interleukin-11, adiponectin, angiotensin, and cathepsin D.
g. ASCs are mesodermal and can differentiate into adipogenic, osteogenic, chondrogenic, myogenic, cardiomyogenic, angiogenic, tenogenic, and periodontogenic lineages.
h. ASC cell surface markers
i. Positive for CD44 (hyaluronate), CD90, integrin β1 (CD29), endoglin (CD105), and integrin α4 (CD49).
ii. Negative for the hematopoietic markers CD45, CD34, and cKit (CD117).
i. In vitro:
i. Isolated from floating portion of lipoaspirate or after finely mincing whole adipose tissue
a) Adipose tissue placed in 0.075% collagenase in Hank’s buffered solution in 37 degree shaking water bath for 1 hour venting every 15 minutes.
b) Collagenase neutralized with 10% fetal bovine serum (FBS) in PBS.
c) Tissue is then spun down in centrifuge.
d) Superior layer of adipocytes aspirated and cell pellet is resuspended in a medium and plated.
ii. ASCs display a cell doubling time of 2 to 4 days, and the medium should be changed every 2 to 3 days.
iii. Differentiation capacity and phenotype are similar to those MSCs isolated from bone marrow and umbilical cord blood.
iv. On average, 45 mL of starting lipoaspirate can be plated onto 1 × 10 cm2 cell culture plates, which can be split to three 10 cm plates 3 days later.
v. Osteogenic differentiation
a) Seed cells appropriately in plate size for desired experiment
1) 30,000 cells/well for a 12-well plate.
2) 100,000 cells/well for a 6-well plate.
3) 800,000 cells for a 10 cm plate.
b) After cells grow overnight in standard growth medium (SGM) (Dulbecco’s modified Eagle’s medium (DMEM) + 10% FBS + 1% penicillin/streptomycin), change media to osteogenic differentiation medium (ODM).
c) ODM: 10 mM glycerol β-phosphate, 0.25 mM ascorbic acid, 10% FBS, 1% penicillin and streptomycin, and DMEM as given below.
d) DMEM: To make 100 × stock solutions (1 M G-2P and 25 mM AA2)
1) 216 g G-2P in 1,000 mL medium or 43.2 g in 200 mL serum-free medium.
2) 4.4 g AA2 in 1,000 mL water or 0.88 g in 200 mL water.
3) Filtrate with 0.22-μm filtration device.
4) Aliquote 10 mL and transfer into 15 mL tube and store at –20°C for up to 3 months.
e) Length of differentiation
1) Human ASCs stain positive for alkaline phosphatase at 3 days and mouse ASCs around 7 days.
2) Bone mineralization by alizarin red can be seen by 7 days in human ASCs and 10 to 14 days for mouse ASCs.
f) Bone morphogenetic proteins (BMPs)
1) Member of TGF-β.
2) Play a significant role in osteoblast differentiation and osteogenesis: BMP-2, BMP-4, and BMP-7.
3) Initiate their signaling cascade through bone morphogenic protein receptor types I and II.
4) These activated receptor kinases subsequently phosphorylate transcription factors Smad 1, Smad 5, and Smad 8.
5) Activate Cbfa1 or Runt-related protein 2 (Runx-2) and OPN as well as stimulate osteogenic differentiation.
6) Runx-2 and Osx are considered the master regulation genes for bone formation.
vi. Adipogenic differentiation
a) Inverse relationship between adipocytes and osteoblasts in bone marrow.
b) Peroxisome proliferators–activated receptor gamma (Pparγ) has been shown to play a key role in adipogenic differentiation.
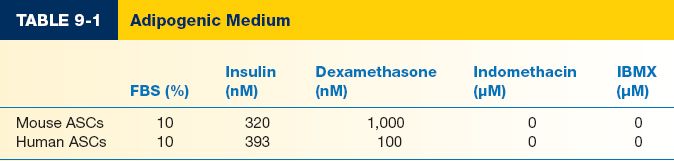
c) Data extremely variable for components and concentration of components. In general, includes DMEM+FBS, insulin, and dexamethasone (Table 9.1). Some will also include indomethacin and IBMX, though more common for bone marrow-derived MSC differentiation.
d) To assess adipogenic differentiation, use Oil Red O stain.
vii. Chondrogenic differentiation
a) Medium: High (4.5 g/L) glucose (DMEM-HG, Invitrogen) supplemented with 10% ITS + Premix Tissue Culture Supplement (Becton Dickinson), 10−7 M dexamethasone (Sigma), 1 μM ascorbate-2-phosphate, 1% sodium pyruvate, and 10 ng/mL TGF-β1.
b) Cells are grown as micromass with 1.25 × 106 cells/mL.
PEARLS
1. When harvesting fat for grafting, larger syringes or plunger-locking devices may create increased pressure that risks damage to tissue.
2. Important to use many small passes for laying fat down to improve vascularity to fat grafts.
3. Once recipient site for fat grafting feels like a large open space, probably it is best to stop grafting.
QUESTIONS YOU WILL BE ASKED
1. What is the 6-month viability of fat grafting?
Around 50%
2. What is the most common complication after fat grafting?
Resorption
3. With fat grafting, why is it important for very small volumes to be injected with each pass?
To promote maximal contact between graft and surrounding bed
Recommended Readings
Coleman SR. Structural fat grafting: more than a permanent filler. Plast Reconstr Surg. 2006;118(3 Suppl):108S–120S. PMID: 16936550.
Gir P, Brown SA, Oni G, Kashefi N, Mojallal A, Rohrich RJ. Fat grafting: evidence-based review on autologous fat harvesting, processing, reinjection, and storage. Plast Reconstr Surg. 2012;130(1):249–258. PMID: 22743888.
Locke MB, de Chalain TM. Current practice in autologous fat transplantation: suggested clinical guidelines based on a review of recent literature. Ann Plast Surg. 2008;60(1):98–102. PMID: 18281805.
< div class='tao-gold-member'>