INFECTIONS
Definition of Terms
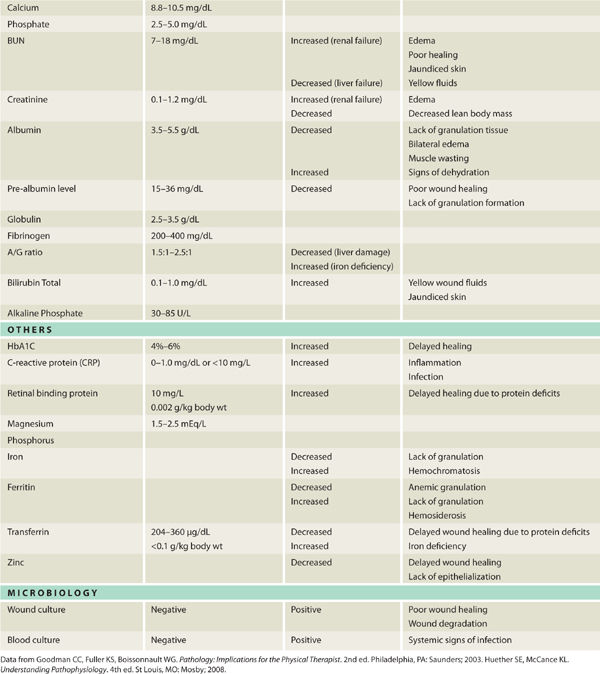
Pathogens, defined as any microorganism that can cause disease in its host, can either damage or infect any wound tissue, or they can adhere to and colonize the wound surface. One distinguishing feature of pathogenic (vs nonpathogenic) microorganisms is their ability to evade the host innate and adaptive immune defenses. Pathogens have developed a variety of strategies to avoid immediate destruction by the host, including covering themselves in a thick polysaccharide capsule termed biofilm,1 or in the case of mycobacterium (eg, Mycobacterium leprae, Mycobacterium tuberculosis), growing inside the macrophage phagosomes while inhibiting the phagosomal acidification and subsequent fusion with lysosomes.2 Bactericidal agents (eg, toxic oxygen-derived products, nitric oxide, AMPs, and enzymes) are released by the phagocytic cells and are intended to render the invader harmless; however, these agents have consequences—they damage the surrounding host tissue and may potentiate the chronic proinflammatory state observed in chronic wounds (see FIGURES 11-2, 11-3).
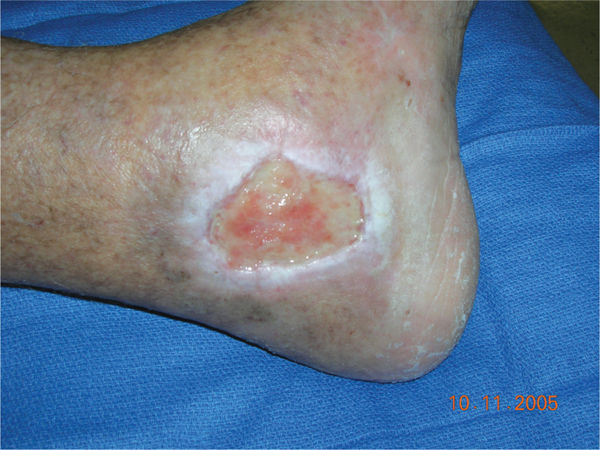
FIGURE 11–2 Infected wound Wound infected with Staphylococcus aureus. The bacteria are not visible; however, the signs of persistent periwound erythema, drainage, epidermal sloughing, and failure to heal are signs of infection. Pain and warmth are other signs of infection that may be present.
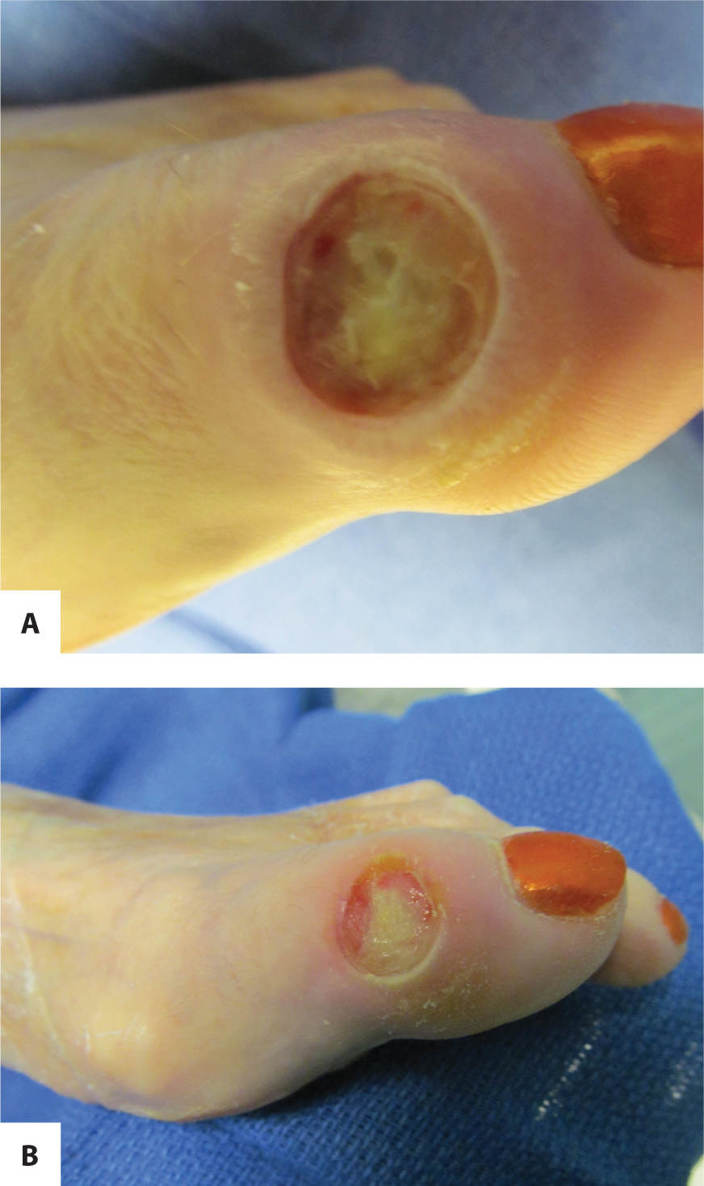
FIGURE 11–3 Infected wound Biofilm, the polysaccharide covering produced on the surface of a wound by bacteria, may not be visible; however, the thick film on the surface of the toe wounds is an indication of possible infection. In this case, the wound culture was positive for MRSA.
Just as the skin is known to have certain flora residing on the surface, so the wound bed has a variable amount of microbial presence that is defined as contaminated, colonized, critically colonized, or infected (see TABLE 11-2). The effect of the microbes on the wound bed is dependent on the type of bacteria, the number of colony-forming units (CFUs) per gram of tissue, and the host immune system. Colonization and critical colonization may respond to topical antimicrobials (See Chapter 13, Wound Dressings); infection and sepsis require that the patient receive systemic antibiotics specific to the invading microbe.
Table 11-2 Terms Defining the Presence of Bacteria on a Wound
Bacterial Infection
The innate immune response is initiated with the engagement of primary host cells and conserved bacterial components, defined as the repetitive arrays of carbohydrates, lipids and proteins that are contained in the bacteria cell walls. These repetitive arrays are termed pathogen-associated molecular patterns (PAMPs) and the receptors that recognize them are termed pattern recognition receptors (PRRs). Host cells (eg, macrophages) constitutively express PRRs that recognize essential and highly conserved microbial components. The PRRs recognize the microbe by contact with its living extracellular components or its phagocytosed components after it has been ingested. Although the PRRs have a limited degree of specificity, they are specific enough to engage the host innate immune system.
Gram-positive bacteria carry a number of proinflammatory cell wall components including peptidoglycan (PGN), teichoic acid, lipoteichoic acid, and other surface proteins. Gram-negative organisms express an extremely potent proinflammatory lipopolysaccharide (LPS). As a result, the evolution of the host innate immune system includes separate and distinct, although sometimes overlapping, sets of sensors to detect the components of both gram-positive and gram-negative organisms. The detection of the organism then sets into motion a cascading proinflammatory response from resident macrophages and leukocytes.1 Bacteria are further characterized as aerobic (needing oxygen to survive) and anaerobic (able to survive without oxygen), and can coexist in chronic wounds, especially if there is necrotic tissue present.3 TABLE 11-3 provides a list of bacteria most commonly found in chronic wounds.
TABLE 11-3 Microorganisms Most Commonly Present in Chronic Wounds
Biofilms
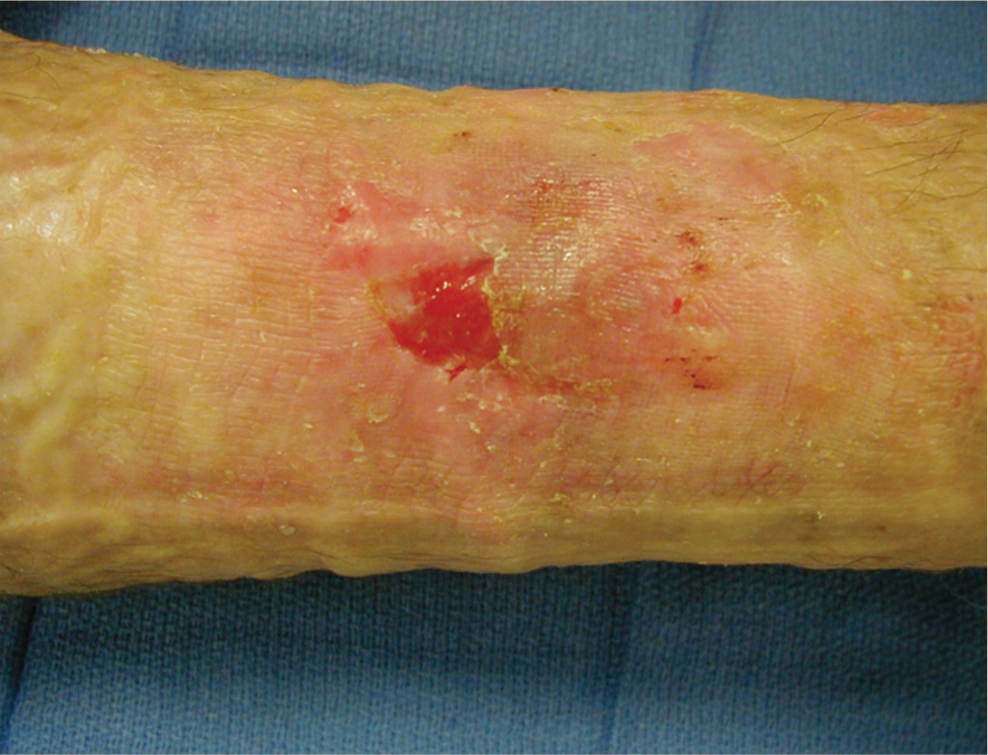
Colonizing pathogens establish an attachment to the host epithelium or wound surface that is composed of an exopolymeric matrix of polysaccharides, proteins, and DNA synthesized by the bacteria.3 The substance is adherent to the wound bed, providing an environment for the bacteria to live and replicate, and thus it is not easily removed by mechanical force or competing bacteria. Studies indicate that the first microorganism to establish host attachment has an advantage over subsequent colonizers even though competition of bacteria often occurs at the level of attachment to host receptors.4 The biofilm can sometimes resemble a layer of slough on the wound surface, or it can be invisible (FIGURE 11-4). In order for the wound to progress from chronic to healing, the biofilm must be removed, preferably with sharp debridement as most antimicrobial dressings will not penetrate the biofilm to kill the bacteria.
FIGURE 11–4 Biofilm on the wound surface Biofilm can appear as a thin, adhered yellow layer on the wound that is difficult to penetrate with antimicrobial dressings. Usually sharp debridement, in combination with contact low-frequency ultrasound or an iodine-based topical dressing, is required to remove the film and attached bacteria.
Fungus Infections
Fungus infections are an increasing problem, especially among patients who are immunosuppressed or have transplants. Recent studies indicate that the fungal response to phagocytosis actively modulates the host immune cell function. Fungal pathogens avoid detection by masking PAMPs (such as cell wall carbohydrates), and by downregulating the complement cascade. Once detected, various species of fungi actually interfere with phagocytosis and can repress production of antimicrobial substances like NO. Some fungi successfully replicate while inside the host macrophage. It is becoming readily apparent that fungi manipulate the host-pathogen interaction to their advantage (FIGURE 11-5).5
FIGURE 11–5 Fungal infection Fungal infections can occur under compression wraps if the skin becomes a supportive moist environment. Note the circular presentation of the infections, which has given some fungal infections the laymen’s nomenclature of ringworm.
In the wound care clinic, they are sometimes observed in conjunction with compression therapy, especially if using dressings and systems that stay in place up to 7 days. The moisture of both wound drainage and perspiration creates an environment where fungi thrive. The infection can usually be managed with a topical antifungal medication, adequate absorbent dressings to manage the wound fluid, and more frequent dressing changes.
Accurate identification of any bacteria or fungi that is impeding wound healing is necessary for effective treatment, and effective treatment is necessary for wound healing to progress.
MEDICATIONS
Steroids
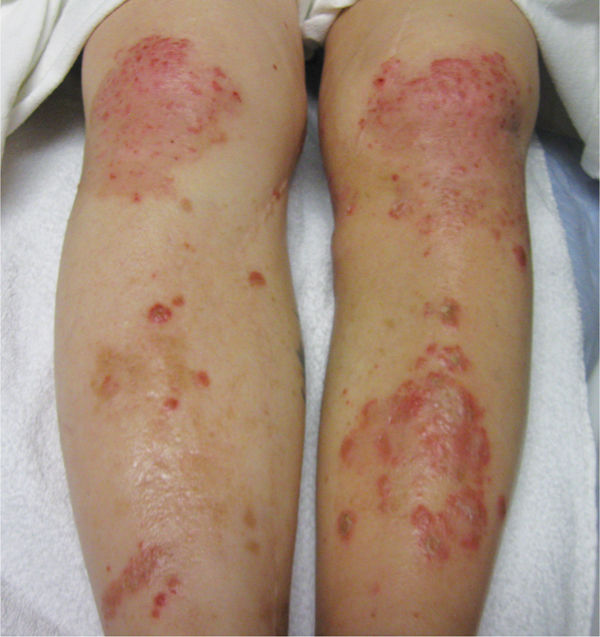
Anti-inflammatory steroids, including glucocorticosteroids, are known to significantly affect many aspects of wound healing. When steroids have been part of the patient’s medication regime prior to wounding, the elevated corticosteroid levels delay the appearance of inflammatory cells and fibroblasts; decrease the deposition of ground substances and collagen; and inhibit angiogenesis, wound contraction, and epithelial migration (FIGURE 11-6A, B).6,7 The effect of steroid-mediated, delayed healing has been demonstrated to occur largely through the downregulation of TGF-β and ILGF1. As reviewed in Chapter 2, TGF-β affects all phases of the healing process (inflammatory, proliferative, and remodeling). The effects of TGF-β on ECM formation are more profound than any other growth factor, and in the absence of TGF-β, matrix deposition and angiogenesis are impaired.8 Specifically TGF-β is mitogenic for fibroblasts and stimulates the production of fibronectin and collagen. Insulin-like growth factor (ILGF-1) is a major regulator of wound healing. Wounds deprived of 90% of their IGFs demonstrate impairment in cell replication and deposition of collagen and a constitutive decrease in wound macrophage numbers. ILGF-1 also directly engages fibroblasts, endothelial and epithelial cells.9,10
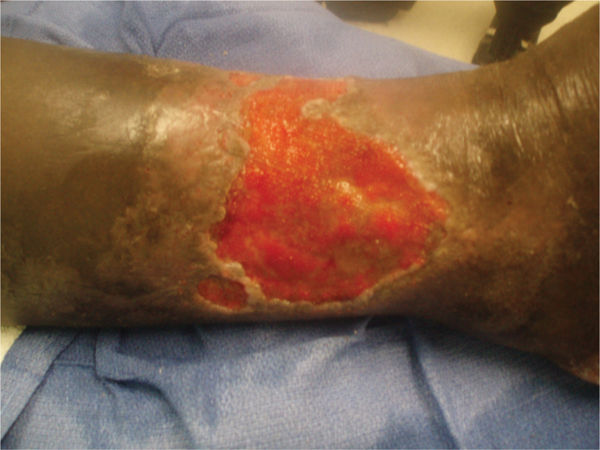
FIGURE 11–6 A. Wound on patient taking steroids The wound on the great toe of a patient who takes steroids for severe rheumatoid arthritis has impaired healing.
B. The wound after 10 days of treatment Debridement of necrotic tissue, low-frequency noncontact ultrasound, and antimicrobial dressings have decreased the periwound inflammation; however, the ability to move from inflammation to proliferation is slow.
The wound healing trajectory is affected in several ways in patients who have been or are currently taking steroids. Decreased leukocyte infiltration, delayed insufficient inflammatory response to injury, and reduced autolysis of necrotic tissue are characteristic of a dampened response early in the healing process. The healing process and clearance of the debris and/or pathogen is inhibited secondary to decreased macrophage recruitment and proliferation. In the proliferative phase of healing the effects of steroids can be observed in the formation of less ground substance, decreased angiogenesis, and diminished fibroblast function, which directly decreases the amount of collagen synthesis. Fibroblasts do not differentiate into myofibroblasts, thus decreasing wound contraction and overall tensile strength. The effects of steroids on each phase of wound healing may lead to vulnerability for ulcer recurrence.11
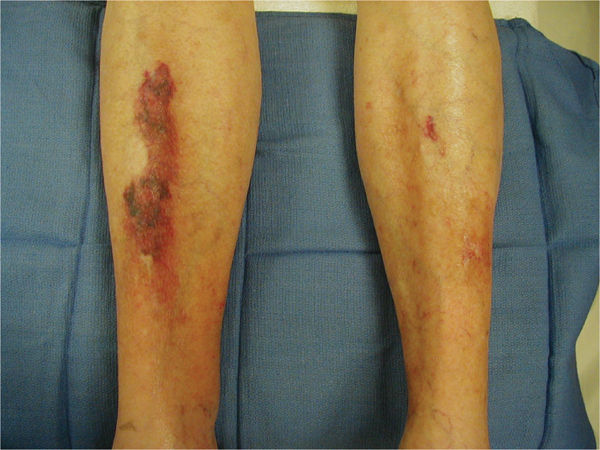
Another potential side effect of long-term corticosteroid use that may affect wound healing is the development of drug-induced diabetes. This condition may not be diagnosed initially; therefore, if a patient receiving corticosteroid therapy has poor wound healing, monitoring blood glucose levels is advised. In addition, patients on steroids develop thin fragile skin that is at risk for skin tears (FIGURE 11-7).
FIGURE 11–7 Skin on patient taking steroids Patients on long-term steroid therapy have thin, fragile skin and tend to bruise easily, thus creating frequent superficial trauma wounds that are difficult to heal. Protection of the extremities with foam sleeves is beneficial, and meticulous wound care is required to prevent infection.
Nonsteroidal Anti-Inflammatory Drugs
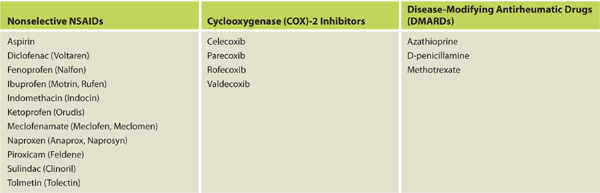
Nonsteroidal anti-inflammatory drugs (NSAIDs) are used to treat both autoimmune diseases and acute injuries because of their ability to decrease inflammation, prevent disease progression, and mitigate pain. (See TABLE 11-4 for a list of commonly used NSAIDs.) These medications work by inhibiting both COX-1 and COX-2 enzymes, thereby decreasing the production of prostaglandins and leukotrienes.12 The positive therapeutic effects of decreased production of prostaglandins are well known; however, the negative effects on wound healing have been studied primarily through animal studies and are less definitive. The mechanisms through which wound healing may be impeded include the following:
Table 11-4 Commonly Used NSAIDs That May Affect Wound Healing
 Decreased production of thromboxane A2, which decreases platelet aggregation and increases the propensity for bleeding and hematoma formation, especially postoperatively
Decreased production of thromboxane A2, which decreases platelet aggregation and increases the propensity for bleeding and hematoma formation, especially postoperatively
 Inhibition of hyaluronic acid production with less granulation formation during the proliferative phase of healing
Inhibition of hyaluronic acid production with less granulation formation during the proliferative phase of healing
 Decreased neutrophil migration to the wound site with increased risk of infection
Decreased neutrophil migration to the wound site with increased risk of infection
 Decreased collagen synthesis with decreased tensile strength of new tissue
Decreased collagen synthesis with decreased tensile strength of new tissue
The results of several studies have led to the recommendation that NSAIDs be discontinued 1 to 4 weeks before surgery, depending on the half-life of the drug being taken by the patient, and some suggest that they should be withheld after surgery until the incision has healed.11,13,14 Many of the studies are based on animal models and results are sometimes inconsistent, so the decision to discontinue their use before surgery needs to be weighed against the effects on the patient’s pain and disease progression.
Some of the clinical signs that may be observed if the NSAIDs are affecting wound healing include failure to progress through inflammation to the proliferative phase, tissue that bleeds easily, poor-quality granulation tissue that will not support reepithelialization, tendency to develop infections, and vulnerability to break down during the remodeling phase (FIGURE 11-8). Patients who are taking NSAIDs for pain relief and not for anti-inflammatory effects may have better healing by substituting acetaminophen, especially if the dosage is high. If patients are on high doses of any anti-inflammatory medications, it is advised that they be tapered off in order to avoid side effects of sudden withdrawal.
FIGURE 11–8 Stalled wound on patient who is taking NSAIDs. The venous wound is clean and granulating; however, it has stalled and is not epithelializing. The patient, who had initially reported only medication for hypertension, told the therapist she was taking 800 mg of ibuprofen each day. Medications were discussed with her primary care physician; she was started pentoxifylline (vasodilator), discontinued ibuprofen, and changed hypertension medications. Within a week the wound had signs of epithelial migration at the edges.
Antirejection Medications
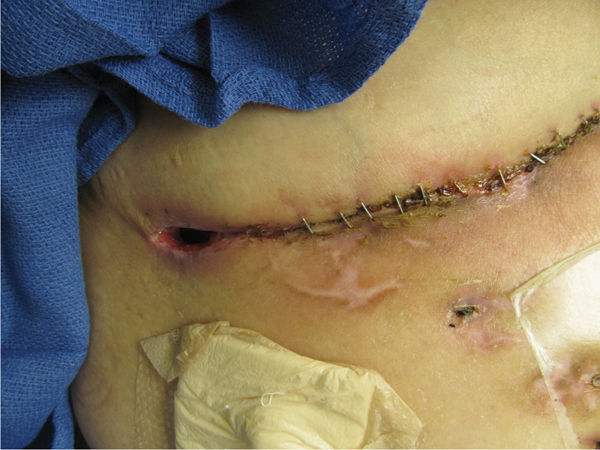
The hallmark of successful organ transplantation is suppressing the immune system so that the host does not activate the immune cells to a level sufficient to reject the allograft. TABLE 11-5 provides a list of commonly used antirejection medications; most of which act by interfering with “discrete sites in the T- and B-cell cascades.”15 Specifically, the actions include inhibition of cytokine transcription, inhibition of nucleotide synthesis, inhibition of growth factor signal transduction, inhibition of the stimulation of T-cell interleukin (IL)-2 receptor sites, and diminished chemotaxis.15 In addition, the effects of the corticosteroids include decreased counts of lymphocytes, monocytes (thus decreased macrophages), and basophils while increasing the number of senescent cells.16 These altered cellular functions are known to impede wound healing, resulting in frequent dehiscence of the transplant surgical incision (FIGURE 11-9).
Table 11-5 Antirejection Medications Used for Organ Transplantation
Data from Lake DF, Briggs AD, Akporiaye ET. Immunopharmacology. In: Katzung BG, Masters SB, Trevor AJ, eds. Basic & Clinical Pharmacology. 12th ed. New York: McGraw-Hill; 2012:chap 55. http://www.accessmedicine.com/content.aspx?aID=55831418. Accessed May 3, 2013.
FIGURE 11–9 Surgical incision on a transplant patient The patient on antirejection medications (Prednisone and Cellcept) after liver transplant. Note the poor epithelial bridging of the approximated edges and the opening at the right side of the incision, indicati ng poor healing of the subcutaneous tissue with tunneling under the incision. The wound may require surgical I&D and negative pressure therapy to promote healing and prevent infection.
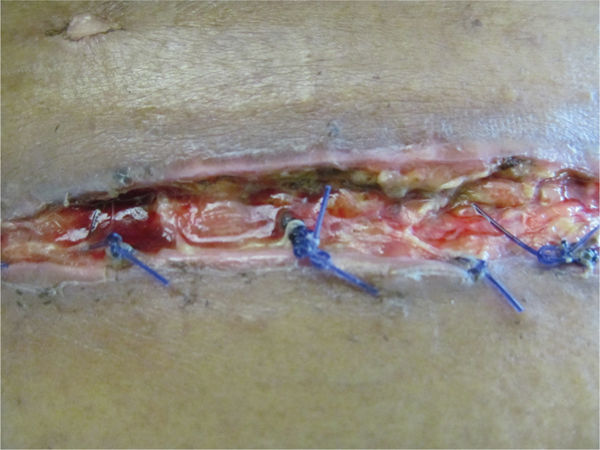
Acute adverse effects of the antirejection medications may include increased risk of infection; nausea, vomiting and diarrhea; and loss of appetite, all of which result in a diminished nutritional state with subsequent insufficient calories, proteins, and vitamins needed for incisional healing (FIGURE 11-10).
FIGURE 11–10 Close view of surgical wound Evidence of protein deficiency is visible in the poor quality of the tissue in this surgical incision, including lack of granulation tissue, slough, rolled edges, and extrusion of the deep sutures, which are acting as a foreign body.
Because antirejection medications are required for the life of the recipient, the effects on wound healing are long term and may result in chronic wounds. This is especially a problem if the patient develops drug-induced diabetes. Another long-term effect of the medications is a significantly higher rate of aggressive squamous cell carcinoma (SCC), a malignant skin cancer linked to the increased number of senescent cells16 and an oxidative environment.17,18 Careful skin inspections are advised in order to detect SCC in the early stages and to increase survival rates.
COMORBIDITIES
Diabetes
Diabetes has multiple inhibitory effects on wound healing, including neuropathic, macrovascular, and microvascular changes, as well as altering cytokine and growth factor signaling. Regardless of the etiology of the wound (surgical, neuropathic, pressure, venous, arterial, acute, or chronic) the patient with diabetes or routinely uncontrolled blood glucose levels is at risk for delayed healing. At the cellular level, diabetes is associated with decreased PDGF receptor expression19 on epithelial and endothelial cells, thereby altering the wound healing process at the initial phase of hemostasis. In addition, patients with diabetes exhibit a prolonged inflammatory phase attributed to the following:
1. A disproportionate expression of vascular cell adhesion molecules by endothelial cells, which increases extravasation and inflammatory cell accumulation at the wound site.
2. The presence and increased infiltration of wound-activated macrophages (WAMs), which contribute to an increased and prolonged expression of inflammatory cytokines.20,21
In the proliferative phase, diabetic wounds exhibit the following characteristics:
1. Increased accumulation of fibrotic ECM at wound edges results in stalled keratinocyte migration (FIGURE 11-11).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


 History of HIV for more than 15 years
History of HIV for more than 15 years History of Kaposi sarcoma on the right anterior leg at the site of the current wound, treated with radiation and chemotherapy (doxol or doxorubicin)
History of Kaposi sarcoma on the right anterior leg at the site of the current wound, treated with radiation and chemotherapy (doxol or doxorubicin) History of recurrent cellulitis, treated with both IV vancomycin and oral Xyvox
History of recurrent cellulitis, treated with both IV vancomycin and oral Xyvox Works as a landscape artist
Works as a landscape artist Spends a lot of time standing and walking
Spends a lot of time standing and walking Is compliant with all medications, takes vitamins
Is compliant with all medications, takes vitamins Works out at the gym 3 to 4 days a week
Works out at the gym 3 to 4 days a week Eats a healthy diet
Eats a healthy diet Has no history of smoking; occasional alcohol
Has no history of smoking; occasional alcohol Has no history of drug abuse
Has no history of drug abuse Wound size: 2 cm × 2 cm
Wound size: 2 cm × 2 cm Friable granulation tissue that bled very easily
Friable granulation tissue that bled very easily Periwound erythema
Periwound erythema Moderate serous drainage
Moderate serous drainage Moderate pitting edema with severe venous reflux
Moderate pitting edema with severe venous reflux 2–3/10 pain with touch or prolonged standing
2–3/10 pain with touch or prolonged standing 3+ dorsalis pedis and posterior tibialis pulses
3+ dorsalis pedis and posterior tibialis pulses No hair growth on the periwound skin
No hair growth on the periwound skin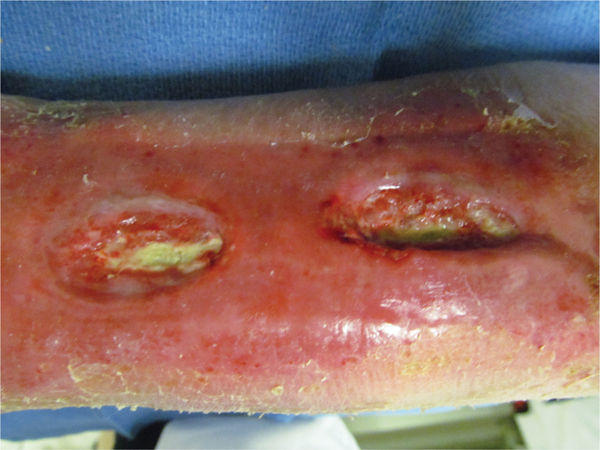
 Contamination—presence of nonreplicating bacteria on the wound surface without any effect upon the wound healing process
Contamination—presence of nonreplicating bacteria on the wound surface without any effect upon the wound healing process Colonization—presence of replicating bacteria attached to the wound surface with no harm to the host and no effect on the wound healing process
Colonization—presence of replicating bacteria attached to the wound surface with no harm to the host and no effect on the wound healing process Critical colonization—presence of replicating bacteria on the wound surface with sufficient numbers to visibly affect the wound healing process
Critical colonization—presence of replicating bacteria on the wound surface with sufficient numbers to visibly affect the wound healing process Infection—presence of replicating bacteria that have invaded the surrounding tissue with visible effects in the wound healing process and in the periwound tissues. Clinical infection for most bacteria is defined as105 CFUs/g of tissue.
Infection—presence of replicating bacteria that have invaded the surrounding tissue with visible effects in the wound healing process and in the periwound tissues. Clinical infection for most bacteria is defined as105 CFUs/g of tissue. Sepsis—presence of replicating bacteria that produces a whole-body inflammatory state termed systemic inflammatory response syndrome (SIRS).
Sepsis—presence of replicating bacteria that produces a whole-body inflammatory state termed systemic inflammatory response syndrome (SIRS). Acinetobacter baumannii
Acinetobacter baumannii Coliforms
Coliforms Enterococcus faecalis
Enterococcus faecalis Methicillin-resistant Staphylococcus aureus
Methicillin-resistant Staphylococcus aureus Pseudomonas aeruginosa
Pseudomonas aeruginosa Staphylococcus aureus
Staphylococcus aureus Staphylococcus epidermidis
Staphylococcus epidermidis Streptococcus pyogenes
Streptococcus pyogenes Bacteroides spp
Bacteroides spp Fusobacterium spp
Fusobacterium spp Peptostreptococcus spp
Peptostreptococcus spp Porphyromonas spp
Porphyromonas spp Prevotella spp
Prevotella spp Veillonella spp
Veillonella spp