AT
BE
BG
CY
CZ
DE
DK
EE
EL
ES
FI
FR
HU
IE
IT
LT
LU
LV
MT
NL
PL
PT
RO
SE
SI
SK
UK
Allowing procurement of hES cells from supernumerary embryos by law
X
X
X
X
X
X
X
X
X
X
X
Specific legislation for human embryo research incl. supernumerary embryos but without specific reference to hES cells
X
X
X
X
Prohibiting procurement of hES cells from human embryos but allowing importation of hES cell lines
X
X
No specific legislation regarding hES cells research
X
X
X
X
X
X
X
X
X
X
Allowing creation of human embryos for procurement of hES cells by law
X
X
X
Prohibiting creation of human embryo for research purpose and for procurement of hES cells by law or by ratification of the convention of the Council of Europe Human rights and biomedicine signed in Oviedo on 4 April 1997
X
X
X
X
X
X
X
X
X
X
X
X
X
X
X
X
X
X
X
X
X
X
Fetal stem cells have intermediate features between embryonic and adult cells [3–8]. They can be isolated from both the fetus and extraembryonic structures of fetal origin and represent a source of stem cells qualitatively better than adult stem cells, despite having a reduced multipotency when compared to embryonic stem cells. They have been used with good results in treatment of patients suffering from Parkinson’s disease but do not have any potential use in therapies in which cells identical to those of the patient are necessary.
Adult stem cells, also called somatic multipotent cells, can be found in differentiated tissues. The natural function of these cells is to replace lost cells in damaged or aged tissues. They can be divided into different groups according to their morphology, cell markers expression, potential of differentiation, and tissue of origin. Some examples are mesenchymal stem cells (MSCs), hematopoietic stem cells (HSCs), and endothelial progenitor cells (EPS). Current studies focus their attention on the ability of these cells to divide and renew themselves indefinitely and, in certain conditions, to differentiate and produce all specialized cell types of their tissue of origin.
Mesenchymal stem cells (MSCs) are non-hematopoietic cells discovered in 1970 by Friedenstein et al. [9], who described a cell population, isolated from animal bone marrow stroma, clonogenic, plate adherent, and capable to differentiate in vitro into bone, adipose tissue, and cartilage [8]. Subsequent studies showed that MSCs resided not only in bone marrow but also in most connective tissues such as peripheral blood [10], adipose tissue [11], trabecular bone [12], dermis [13], synovial membrane [14], synovial fluid [15], tendons [16], skeletal muscle [17], and blood, liver, and fetal bone marrow [18, 19]. In addition to their spread and the relative simplicity of isolation, MSCs are candidate to be used in regenerative therapy for their high proliferation rate and the possibility of differentiating into cells capable of replacing a damaged tissue [20, 21]. Furthermore, MSCs have a low immunogenicity and low percentage of patients with GVHD (graft versus host disease) following transplantation, due to their capacity to suppress lymphocyte reaction [22]. MSCs injected by intravenous infusion are able to migrate to damaged site and differentiate into appropriate phenotype required for tissue repair or secrete growth factors and cytokines that favor, through paracrine and autocrine activity [23], HSC proliferation. Despite MSCs from bone being the first to be isolated and studied, their collection is invasive and only allows a small amount of sample to be obtained. The small size of the sample is also a limitation in taking of MSC from umbilical cord blood. For these reasons, there is a growing interest in MSC isolation from the skin that represents a more easily accessible source and allows obtaining a good amount of cells.
The skin is the largest human organ (its size varies from 1.3 to 2 m2), and it has a thickness of 3–6 mm, maximum at feet soles and hands palms. It is a constant renewal organ, and it contains a series of cellular populations that originate both from mesoderm and ectoderm. Recently, the skin has drawn the attention of scientists as a reservoir of different stem cells [24, 25]. It has been demonstrated that progenitor cells of dermis and follicle are able to differentiate in vitro into neurons [26], smooth muscle cells, melanocytes, chondrocytes, and Schwann cells. Subsequent studies showed that stem cells derived from the skin (S-MSCs) can differentiate in vitro into adipocytic, osteogenic, and chondrocytic lines and that their membrane markers are very similar to MSCs derived from bone marrow [27]. S-MSCs were isolated from foreskin [28], follicle and bulb hair [29], biopsies of healthy skin taken from adult volunteers, and scalp [30]. Several studies have confirmed that S-MSCs have properties common to all stem cells: they show ability to proliferate for many passages in culture maintaining a relatively unspecialized phenotype and, under specific conditions of culture medium, to differentiate into specialized cellular types [31].
As previously described, it is possible to isolate S-MSCs from skin biopsies of about 1 cm2; skin samples must be deprived of the subcutaneous tissue, divided into small fragments, and positioned on the bottom of a well plate for cell cultures with a mesenchymal stem cell basal medium enriched with growth supplements for the mesenchymal stem cells, l-glutamine, and penicillin/streptomycin (mesenchymal stem cell growth medium – MSCGM). After approximately 8 days of culture, some adherent cells appear near to the explants (Fig. 23.1). Nonadherent cells must be removed and the medium changed twice a week. Reached confluence, cells have to be detached from the plate and transferred in increasing size flasks. In agreement to minimal criteria for identification of human MSCs established by Dominici et al. [32], the immunophenotype of the isolated cells must be analyzed by staining with fluorescein isothiocyanate (FITC)-conjugated antibodies against HLA-DR, CD14, CD19, CD34, CD45, CD73, CD90, and CD105 (Table 23.2). In addition, the gene expression of mesenchymal markers (e.g., Oct-4, Thy-1, or c-kit) and markers of differentiated cells (as, e.g., keratin-1) is tested by using real-time PCR. Finally, to assess the stemness of isolated cells, the differentiative potential into chondrogenic, osteogenic, and adipogenic cells must be proved.
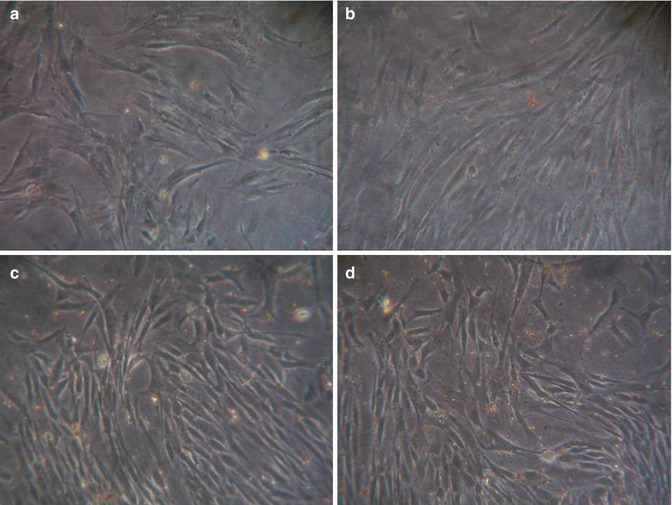

Fig. 23.1
Phase contrast microscopy image gallery showing S-MSC morphology during their culturing. Panel (a), early MSC-like cells after the explant; panel (b), nonuniform population with cells in suspension soon after the earliest passage; panel (c), homogeneous, confluent cell monolayer with fibroblastoid morphology after 14 days of culture; panel (d), cells that acquired stellate morphology after 12 weeks culturing. Sections (b) and (c), bar = 100 μm; Sections (a) and (d), bar = 200 μm
Table 23.2
Flow cytometry results of MSCs
HLA-ABC | + |
HLA-DR | − |
CD14 | − |
CD19 | − |
CD34 | − |
CD45 | − |
CD73 | + |
CD90 | + |
CD105 | + |
However, methods and culture media conditions required to obtain different types of S-MSCs for potential clinical applications need further studies. For example, the age and gender of donor could affect responses to growth conditions of cell cultures [33]. In order to obtain optimal procedures of replication of S-MSCs, it is necessary to analyze the replication rate in different culture conditions, the secretion of certain growth factors, and the expression of surface markers [34, 35]. In vivo stem cells reside in a specific tissue microenvironment (stem cell niche) in which they receive extrinsic signals necessary to maintain their undifferentiated phenotype. Culture media and supplements are critical factors that can stimulate or modulate the proliferation of stem cells in vitro. A recent study by Jeon et al. shows that medium conditioned by MSCs stimulates substantially the survival of fibroblasts and induces the production or secretion of collagen, elastin, and fibronectin [36




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







