Abrams ML, Smidt A, Benjamin L, Chen M, Woodley D, Mancini AJ. Arch Dermatol 2011; 147: 337–41.
Epidermolysis bullosa acquisita
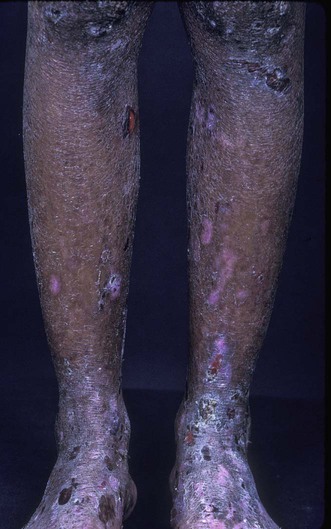
Specific investigations
Congenital epidermolysis bullosa acquisita: vertical transfer of maternal autoantibody from mother to infant.
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree





