CHAPTER 16 Enucleation, Evisceration, and Exenteration
The Care of the Eye Socket
When to remove the eye
The scleral shell
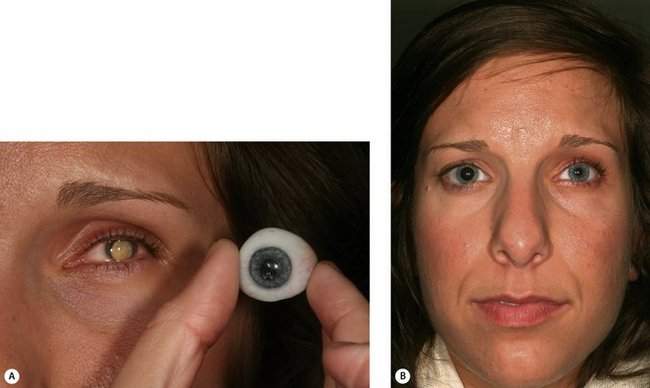
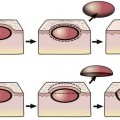
When a patient comes to you with a blind eye requesting removal of the eye, the first question to ask is, “Does the eye cause you pain?” If the answer is no, consider a scleral shell. A scleral shell is a thin ocular prosthesis that fits over the blind eye (Figure 16-1). The shell provides a natural appearance and is comfortable for most patients. No surgery is required to treat the patient. Patients who are ideal candidates for wearing a scleral shell are those with:
Patients with severe pain probably will require enucleation or evisceration. You can sometimes treat patients for the mild aching pain that may accompany phthisis with prednisone ophthalmic (Pred Forte 1%) and atropine drops to control any intraocular inflammation. Undoubtedly, your enthusiasm for a scleral shell decreases as the complexity of any treatment to minimize pain increases. For many patients, removal of the eye is better than long-term treatment with drops and a shell, but at least consider the option of controlling mild pain medically. Most patients can tolerate the fit of a shell even with normal corneal sensation. Although the scleral shell sits over the eye, the posterior face of the shell is vaulted to sit off the cornea. If there is any question about pain, consider a trial period with a custom conformer. This prosthesis is custom-made to fit the shape of the socket, but does not have any color applied. The cost of the custom conformer is only 10–20% that of a completed prosthesis, so a trial is worth the cost if doubt exists that the shell will be tolerated. Although the scleral shell can be thin, an element of enophthalmos or phthisis makes it easier to get symmetry (Figure 16-1). Don’t recommend fitting a scleral shell if you think that the eye is continuing to shrink. In a few months, the shell will not fit and time and money will have been wasted. Obviously, enucleation should be considered for an eye with an intraocular tumor. Most patients wearing a scleral shell are blind in the eye that is covered. A patient with normal vision cannot see through the shell. For any patient with a disfigured iris who has good vision, a cosmetic contact lens (a special contact lens painted with matching iris details) should be considered.
Enucleation or evisceration?
Once the decision has been made to remove the eye, there are two options:
The indications for enucleation are:
Evisceration does not permit a complete, controlled removal of an intraocular tumor. Surgical margins are impossible to evaluate. Eyes that are shrunken as a result of phthisis should not undergo evisceration because the sclera cannot hold an adequate-sized implant.
You should know that many of my trusted colleagues have switched to evisceration whenever possible.
The enucleation operation
Preoperative considerations
Choice of implant
The term integrated implant was originally used to describe the attachment of a prosthesis to the implant. As was stated earlier, exposed implants were integrated directly to the prosthesis through an opening in the conjunctiva. This integration provided excellent motility of the ocular prosthesis. Experimentation with different shaped implants led to the development of the quasi-integrated implant (the Iowa and the universal implants). These buried implants had an irregular anterior surface, projecting mounds on the surface of the conjunctiva. An ocular prosthesis was fit with impressions on its posterior surface to “mesh” or “quasi-integrate” with the prosthesis. These implants provide good motility, but they require more time to place and are sometimes associated with migration or rotation of the implant.
There is no perfect anophthalmic socket implant. Everyone agrees that the implant should restore adequate orbital volume requiring at least a 20 mm sphere. Most surgeons would agree that the extraocular muscles should be reattached to the implant. There are differing opinions regarding whether porous or solid implants are best. My current personal choice for an ocular implant is a 20 mm MEDPOR SST sphere. This implant has predilled holes that allow easy attachment of the muscles, and the anterior surface of the implant is smooth, so that exposure is an infrequent problem (MEDPOR PLUS SST porous polyethylene spheres: 16 mm #80046, 18 mm #80048, 20 mm #80050, 22 mm #80052, http://www.porexsurgical.com). We use no integration of the implant and the prosthesis. Movement is good in the conversational range (within 10–15 degrees of primary position).
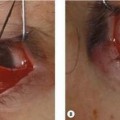
Enucleation operation
Enucleation procedure
The enucleation procedure using a MEDPOR SST spherical implant includes:
The steps of the enucleation operation with placement of a MEDPOR PLUS SST spherical implant are:
2. Detach the extraocular muscles
5. Attach the muscles to the implant
6. Close Tenon’s capsule and the conjunctiva
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree