TOGa system ® (Satiety Inc.)
Incisionless Operating Platform ®(USGI Medical)
Endoscopic suturing device (Wilson-Cook Medical)
Stomaphyx ® (EndoGastric Solutions)
Anubis (Storz)
SafeStitch (SafeStitch Medical, Inc.)
Endo–Cinch and RESTORe SuturingSystem™ (C.R. Bard Inc.)
Spider (Transenterix)
NDO plicator ® (NDO Surgical, Inc.)
TERIS (Barosense Inc.)
Endosamurai (Olympus)
Overstitch (Apollo Endosurgery, Inc.)
Spiderman (Ethicon Johnson & Johnson)
DDES – Direct Drive Endoscopicsystem (Bard, Inc.)
Some endoscopic suturing devices (ESDs) were primarily developed, years ago, for endoluminal treatment of gastroesophageal reflux disease (GERD). Being capable of applying a partial thickness suture on the EGJ, their previous goal was to create a fold at the EGJ to reduce reflux. Results have been controversial and largely debated [17].
They have been recently reintroduced in bariatric surgery to produce a gastric plication of stomach layers (mucosal and submucosal) with the aim of reducing gastric volume, and then performing a trans-oral gastroplasty.
The first reported device used was the EndoCinch Suturing System® (Davol Inc.). It is inserted over a standard endoscope and fires a straight threaded needle through a tissue fold obtained by suction. A series of sutures can be placed in two rows in a full thickness manner [18, 19].
In 2008, Fogel et al. reported a series of 64 obese patients treated by means of an endoscopic vertical gastroplasty with a 1-year follow-up. They registered a significant reduction in BMI at 12 months (mean BMI 39.9 ± 5.1 kg/m2 vs 30.6 ± 4.7 kg/m2) and %EWL of 21.1 ± 6.2, 39.6 ± 11.3 and 58.1 ± 19.9 at 1, 3 and 12 months, respectively, comparable with the control group treated by mean of LRYGB [20]. Fogel’s excellent results have not been achieved in other published trials.
15.3.1.1 TOGA System
Promising results have been reported for a series of patients treated by means of TOGA system (Satiety Inc.), used to create a stapled restrictive pouch along the lesser curvature of the stomach [16].
The TOGA system has two disposable stapling devices: the TOGA Sleeve Stapler and the TOGA Restrictor. The first is used to create a vertical sleeve along the lesser curvature of the stomach, approximately 8 cm in length and 2 cm in diameter. The second is used to reduce the sleeve outlet size by creating folds at the distal end of the pouch created [21].
The procedure consists in the introduction of a 60-French bougie through the mouth and oesophagus over a guide wire, to dilate and test for any resistance. The TOGA Sleeve Stapler is then introduced over the guide wire and a small endoscope is routed through a channel in the device. Once the stomach cavity is reached, a septum with attached retraction wire spreads and orients the stomach tissue, while suction is applied from the vacuum pods included in the device. Three rows of titanium staples are usually delivered to create a staple line that connects the anterior and posterior stomach, beginning 1 cm proximal to the Z line and extending distally 4.5 cm, parallel to the lesser curvature [22]. According to the latest method tested, a second staple line is added distally. The result of this procedure is a small, restrictive pouch along the lesser gastric curvature [21].
The first human multicentre study in 2007 enrolled 21 patients and reported good results in terms of safety (no serious adverse events occurred) and EWL of 16.2 %, 22.6 % and 24.4 %, respectively, at 1, 3 and 6 months. However, this first study showed a possible flaw of the procedure, since gaps in the staple line were evident in 13 of the 21 patients. At that time, only one staple line was created [23]. Following this first trial, an Italian study analyzed the effects of TOGA on insulin sensitivity and secretion on a series of nine patients. Insulinemia was significantly reduced at fast and at 120′ after OGTT, as well as the insuline secretion rate (from 235.05 ± 27.50 to 124.77 ± 14.50 nmol/min/m2) while insulin sensitivity increased (from 348.45 ± 20.08 to 421.18 ± 20.84 mL/min/m2). Total insulin secretion rate was demonstrated to correlate with weight, fat mass and BMI [22]. A second prospective, multicentre, single-arm trial with a 1-year outcome ended in 2011 and involved 53 patients. A second staple line was added distally, to prevent possible gaps. Nevertheless, 7 patients had a gap on the proximal part of the staple line, 16 had gaps between staple lines and 2 had a combination of gap types; this replicates the same complication of Masons’s gastroplasty which lead to the McLean modified technique. Gap presence negatively correlates with procedure efficacy, remaining an unsolved problem of the TOGA procedure, since no special treatments or salvage techniques are currently available. Patients with no or small gaps (<15 mm) had a responder rate of 87.5 % whereas patients with large gaps (>15 mm) had a rate of 45.5 %. There were only mild side effects related to the procedures, such as nausea, vomiting, abdominal pain, throat pain and dysphagia, which resolved within 1 week. After the post-procedural routine radiographic control, one patient was diagnosed with asymptomatic pneumoperitoneum, which resolved without further complication. This second TOGA trial confirmed the promising results of the first, showing a %EWL of 29.3 ± 11.6 % at 3 months, 36.8 ± 15.7 % at 6 months and 38.7 ± 17.1 % at 12 months. Improvements in the metabolic profile were also reported: HbA1c levels decreased from 7.0 % at baseline to 5.7 % at 12 months for nine diabetic patients. HbA1c levels decreased from 5.9 % at baseline to 5.4 % at 12 months for all others. Triglyceride levels decreased from 142.9 mg/dL at baseline to 98.0 mg/dL at 12 months [21].
15.3.1.2 RESTORe Suturing System
The RESTORe Suturing System™ is a new generation suturing device by Bard Inc., characterized by single-intubation, multistitch capability and designed to place sutures through the muscular wall of the stomach and approximate gastric tissue. The suturing system is activated by depressing a plunger on the top of the device. When activated, the system deploys a 3-0 polypropylene suture through the tissue in the suction chamber and deposits the suture tag in the end of the capsule [24].
The results of a very first trial have been presented at the XVI World congress of the IFSO 2011 by P. Schauer after 12 months of follow-up. Eighteen subjects with a BMI 30–45 kg/m2 (mean 38.6 kg/m2) underwent a gastric volume reduction procedure, each patient required four to eight plications (mean 6), and the average procedure time was 125 min. No serious or significant procedure-related complications have been observed. They report a EWL >30 % for half of the patients and an EWL 30.5 ± 16.8 % for subjects with a BMI between 30 and 35 (TRIM trial investigators, personal communication). The RESTORe is currently being evaluated in the TRIM (Transoral Gastric Volume Reduction as an Intervention for Weight Management) trial.
15.3.1.3 POSE Procedure
A new, less invasive surgical option performing gastric plication is the POSE procedure, inspired by the endoscopic technique used to treat pouch dilation after bariatric surgery, named ROSE procedure. Primary Obesity Surgery Endolumenal (POSE) is a new scar-free technique based on the use of the Incisionless Operating Platform® (IOP® – USGI Medical) made of three main components: TransPort Multi-lumen Operating Platform®, a flexible endoscope with four working channels functioning as flexible trocars, with insufflation capability, and allows operators to deploy and use up to three tools simultaneously. The Platform further consists of a g-Prox® tissue grasper, a flexible endoscopic tissue approximation device deployed via one of the four working channels; Expandable Tissue Anchors®, suturing technology designed to overcome the problems of endolumenal suturing in the GI tract by distributing forces over large tissue contact. These anchors also have a semi-compliant nature that accommodates postoperative swelling. Their plasticity allows them to withstand swelling, yet maintain hold on tissue.
To perform POSE, surgeons advance the USGI TransPort® down the oesophagus to the stomach fundus, like a traditional endoscope. The tip of the device is then turned towards the operating site and locked for stabilization. Once the platform is in place, the flexible grasper is delivered to the operative site through a lumen of the TransPort® and is then used to bite a fold of the stomach mucosa on which the anchors are put (Fig. 15.1). Each anchor pair is preloaded into a catheter (g-Cath®), and inserted into the grasper before firing. Several folds are created in this way to reduce gastric fundus and limit its ability to expand. Once the desired capacity is reached, the device is removed through the oesophagus. POSE is reported to be performed successfully in North America, Europe and the Middle East. From 2011 to 2012, a prospective observational study was undertaken by J. C. Espinós et al. involving 45 patients: 75.6 % female; mean age 43.4 ± 9.2 SD (range 21.0–64.0). At baseline: mean absolute weight, 100.8 ± 12.9 (75.5–132.5); BMI 36.7 ± 3.8 (28.1–46.6). All POSE cases were performed with no intraoperative adverse events, no conversions or failed procedures. No mortality was reported; only two minor postoperative adverse events resulted: one case of low-grade fever (resolved with oral antibiotic treatment) and one patient returned to hospital on the second postoperative day with chest pain. Minor postoperative side effects included sore throat, stomach pain, nausea and chest pain. Three cases of vomiting resolved within the first 12 h with no requirement of additional hospital stay. To be noted, all patients were discharged from hospital in less than 24 h. Liquid intake began 12 h post-procedure with full solids by 6 weeks. Follow-up visits were performed in a tight schedule at 1, 2 and 3 weeks, and at 1, 2, 3, 4, 5 and 6 months. The mean 6-month POSE TBWL was 15.5 % and more than 80.0 % of POSE patients had achieved ≥25 %EWL. The overall POSE patient mean EWL was MLT 40.0 % (calculated by metric) and 49.4 % (calculated with BMI 25 as ideal end point). The authors also report that patients in the current POSE cohort who have reached the 9- and 12-month time points have continued their weight-loss trend without complications [25].
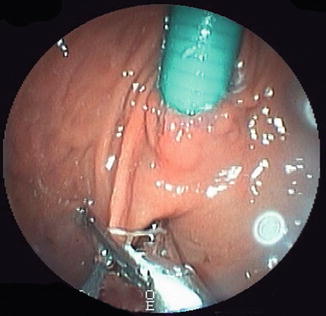

Fig. 15.1
Creation of a gastric fold using IOP® (USGI Medical, Inc.)
15.3.1.4 Overstitch
Similar in concept, the recently redesigned Overstitch, by Apollo Endosurgery, is able to perform transoral endoscopic gastric volume reduction by a series of endoluminally placed, full-thickness, closely spaced interrupted sutures through the gastric wall from the prepyloric antrum to EGJ (Fig. 15.2). Although very fashionable, published data on the procedure is scarce at the time of writing. Among the preliminary experience, Abu Dayyeh in Rochester (Minnesota, USA) performed a transoral endoscopic gastric volume reduction with Overstitch in four subjects. Post-procedural abdominal pain and nausea developed in three patients, while acid reflux symptoms developed in one and resolved with an oral proton pump inhibitor [26]. No data on EWL or TBWL were reported, being only a pilot feasibility study. Long-term safety and efficacy of the procedure are yet to be evaluated.
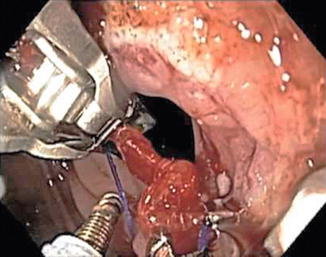

Fig. 15.2
Overstitch® Endoscopic Suturing System (Apollo Endosurgery, Inc.)
15.3.1.5 TERIS
The Trans-oral Endoscopic Restrictive Implant System (TERIS® – Barosense Inc.) uses an endoscopic guidance to trans-orally implant a prosthesis placed at the cardia level to decrease food reservoir size at the upper part of the stomach to induce early and prolonged satiety. Five gastric plications are created about 3 cm below the EGJ using an articulating endoscopic circular stapler. The gastric restrictor is subsequently attached to the gastric wall using silicon anchors inserted through the gastric plications. Primary clinical results, observed in a female patient with a BMI of 46 kg/m2, showed no major complications during and after the procedure. The EWL was 21 and 26 % at 3 and 6 months, respectively. The observation of a high rate of implant obstruction led to the development of a second-generation implant [27].
Later, Fockens et al. published a study on safety and efficacy using the TERIS implant. This short-term trial included 13 patients with comorbidities (BMI 40–50 kg/m2 or 35–40 kg/m2) and a 3-month follow-up. Results reported one patient with intraprocedural gastric perforation related to stapler malfunctioning. Two other patients had a pneumoperitoneum treated conservatively in one case and deflated by a percutaneous hollow needle in the other. Twelve of 13 patients had a successful implant placement. At 3 months of follow-up, a median excess weight loss of 28 % and a median BMI decrease of 4.2 kg/m2 (from 42.1 to 37.9 kg/m2) were reported [28].
15.3.2 Derivative Procedures
A device, which applies a concept similar to the biliopancreatic diversion (BPD), is the duodeno-jejunal bypass liner (DJBL), a sterilized, single-use endoscopic device, which is minimally invasive and used under radioscopic control. It is composed of a nitinol anchor with tiny lateral barbs for fixation, and an impermeable plastic conduit made of a fluorine polymer 62 cm in length (Fig. 15.3), which prevents contact of bile–pancreatic secretions with chime prior to the proximal segments of the jejunum [29].


Fig. 15.3
Duodenal-jejunal bypass liner EndoBarrier® (gi-Dynamics, Inc.) from: http://www.gidynamics.com/
15.3.2.1 Results
The first human experience was registered in 2007 by L. Rodriguez-Grunert et al. with a 12-patient prospective, open-label, single-centre, 12-week study. They reported problems with the fixation of the device and adverse events related to the device itself, including abdominal pain, nausea, vomiting, mainly within 2 weeks of implantation. Implant site inflammation was found in all the patients [30].
Gersin et al. carried out an open-label, sham-controlled trial on obese patients to test weight loss before bariatric surgery due to the implantation of DJBL. The primary end point of the trial was the difference between the two groups, in percentage of excess weight loss (EWL) at week 12. Thirty-seven patients were enrolled in the trial, 13 received the DJBL and 24 patients in the sham group underwent EGD and mock implantation. After 12 weeks, they observed a statistically relevant difference of EWL between the two groups: 62 % of patients in the DJBL achieved 10 % or more EWL compared with 17 % of the subjects in the sham arm. Total weight change in the DJBL arm was −8.2 ± 1.3 kg compared with −2.1 ± 1.1 kg in the sham arm (P < 0.05) [31].
In 2009, Gersin et al. published a study on the use of DJBL for type 2 diabetes treatment. They randomized 18 obese diabetic patients in two groups: the first (12 patients) underwent DJBL implantation while the other (6 patients) underwent sham endoscopy. The subjects baseline HbA(1c) was 9.1 ± 1.7 % and body mass index 38.9 ± 6.1 kg/m2




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







