Fig. 14.1
BIB intragastric balloon (BIB®, Allergan Inc., Irvine, CA, USA) (left); device placed in the stomach (right)
On the market there are, nowadays, other types of intragastric balloons, including the ‘Heliosphere bag’ (Helioscopie, Vienne, France) [4], a balloon filled with air and inducing a minor discomfort compared to that induced by liquid-filled balloon, which has been proved to be effective in treating short-term obesity (Fig. 14.2).
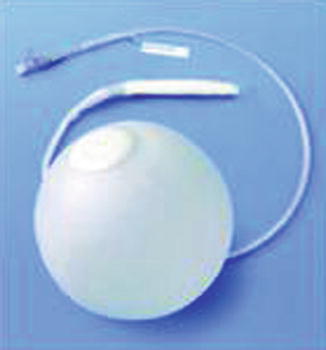

Fig. 14.2
Heliosphere intragastric ballooon (Helioscopie, Vienne, France)
It should also be mentioned the Endogast (Adjustable Totally Implantable Intragastric Prosthesis,ATIIP) (Districlass Medical, France), placed by a combined endoscopic surgical procedure, filled with air and attached to the abdominal wall [5]. It is currently used in few centres (Fig. 14.3).
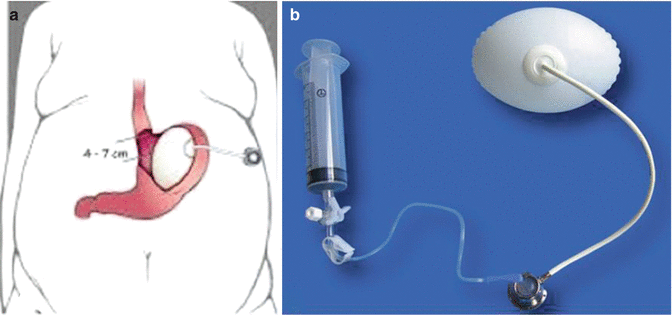

Fig. 14.3
Endogast (Adjustable Totally Implanted Intragastric Prosthesis, ATIIP) (Districlass Medical, France). (a) Schematic illustration of the device, (b) the device
The Obalon® Gastric Balloon (OBG) (ObalonTherapeutics, Inc., San Diego, CA, USA) is a non-surgical and fully repeatable and reversible weight loss device. Compared with other commercially available intragastric balloons, the Obalon is totally swallowable and does not need endoscopy for its placement. Up to three devices can be placed together [6] (Fig. 14.4).


Fig. 14.4
The Obalon® Gastric Balloon (ObalonTherapeutics, Inc., San Diego, CA, USA) as it appears deflated (a), (b) the inflation system gauge to remotely inflate the device, (c) inflated device
14.2 Intragastric Balloon: Mechanism of Action
The efficacy of the device in inducing weight loss is due not to a placebo effect but to the characteristics which make it effective ‘in itself’. How it actually works chiefly depends on its characteristics:
Its weight (if it is filled with liquid), which stimulates the baroceptors at the gastric wall level. These, through the brain–gut axis, stimulate the satiety centre located at hypothalamic level.
Delayed gastric emptying: an ultrasonographic study highlighted the fact that in patients without the balloon, food could already be visualized in the antrum 32 min after the consumption of a solid meal. Thirty days after BIB® placement in the same patients, this study visualized food after 300 min.
Reduction of the unoccupied gastric volume by about 750 cm3 due to the presence of the device.
Discomfort: the overall disorders (nausea, precocious sense of satiety, vomiting, epigastric pain) suffered during the first 24–30 h post-placement and if the patient fails to adhere to the prescribed dietary regime.
Hormonal mechanisms: during treatment with the BIB® in the first 3 months a significant increase in the plasmatic ghrelin levels was observed, followed by a gradual reduction until the basal levels reached after removal.
14.2.1 Indications
The balloon system is indicated for temporary use associated with a specific diet treatment in patients with a history of obesity (at least 5 years), after numerous failures of the dietary treatment only.
The balloon is indicated in patients with:
<35 BMI with obese-related comorbidities whose resolution or improvement requires mandatory weight loss
>35 BMI, as a pre-surgery role in patients with comorbidities, before any other type of surgery, or in patients who refuse surgery
14.2.2 Placement and Removal Technique
Balloon placement and removal can be performed in conscious sedation with diazepam or midazolam, in unconscious sedation with propofol or with orotracheal intubation. Before placement, a diagnostic oesophago-gastro-duodenoscopy is performed.
The BIB balloon is then positioned with the valve under the cardia and is filled, under endoscopic vision, with 500–700 ml of physiological solution and 10 ml vital staining solution (methylene blue). The connection catheter is removed and the valve checked for possible leaks. The mean duration of the procedure is 12 min. Balloon removal is carried out after 6 months. The removal procedure should be preceded by a 72-h, no-‘roughage’ diet and by a 24-h semi-liquid diet. The procedure foresees a gastroscopy to see the balloon and subsequently deflate it with a specific device. The BIB® is removed with a dedicated ‘grasper’ when completely deflated. Stomach observation is necessary to exclude possible mucosal lesions.
The Endogast is placed by a combined endoscopic surgical procedure, filled with air and attached to the abdominal wall. The device is connected to a subcutaneous system to adjust the volume of air.
For the Obalon, the patient swallows a capsule attached to a micro-catheter (the balloon is inside this gelatin capsule). Once in the stomach, verified by fluoroscopy and by the inflation system gauge, the balloon is remotely inflated with gas (nitrogen). After the inflation the micro-catheter is detached and removed, leaving the balloon in the stomach.
After a 3-month treatment period, all the balloons are retrieved by an upper GI endoscopy, using standard, commercially available endoscopic tools.
14.2.3 Post-placement Pharmacological Treatment
Due to the secondary effects deriving from the presence of the balloon (nausea, regurgitation or vomiting, cramp-like epigastric pains) and from the almost total impossibility of eating during the first 24–36 h, all patients must receive support treatment consisting in the infusion of electrolytic solutions, proton pump inhibitors, antispasmodic and antiemetic drugs.
14.2.4 Discharge
Before discharge the patient is made aware of the importance of optimum hydration and of ongoing urine checks (if methylene blue has been used), in order to diagnose in time the premature rupture of the balloon or a possible valve leak in a case a BIB has been placed.
On the first day, the patient receives a liquid diet only. From the second and up to the sixth or seventh day, a semi-liquid diet is followed.
14.2.5 Follow-Up
The dietetic regime from the seventh day is a daily intake of 1,000–1,200 kcal consumed over three main meals and two snacks. This dietetic regime is maintained until removal of the device. When there are signs (e.g. blue urine for the BIB) or symptoms indicating a possible complication, an immediate clinical evaluation of the patient is essential. Decubital ulcers or gastrectasia indicates the need to remove the balloon.
All the patients are informed of the increased chance of balloon rupture if it remains in the gastric cavity for longer than the prescribed period.
At the end of the treatment, the device will, in any case, be removed. The following alternatives are then evaluated: (a) starting the patient of on a ‘maintenance’ diet programme; (b) subjecting him/her to the consensual placement of a second balloon (multiple treatment); or (c) performing the previously planned bariatric surgery.
14.3 Results
14.3.1 Early Secondary Effects
In our experience with the BIB, the secondary post-placement effects were the following: nausea for 24–36 h in 87 % of the patients; vomiting (a mean two episodes) in 51 %; slight epigastralgia in 61 %, regressed with antispasmodic drugs; increased intestinal meteorism in 36 %; diarrhea (5–6 episodes/day) in 5 %; and halitosis in 12 % .
For the Heliosphere, nausea and vomit were present in 7.4 % of the patients during the first week.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







