Fig. 22.1
Superior uncinectomy
Once this step is completed, visualization of the natural maxillary ostium should be possible with the use of an angled scope (Fig. 22.2). Proper identification and inclusion of the natural ostium in the surgically created antrostomy is of critical importance [39]. Creation of posterior antrostomy will result in a dual ostial configuration resulting in mucus recirculation and predispose to recurrent infections. If an accessory ostium is already present, it is joined to the natural ostium using through-cutting instruments. If an infraorbital or Haller cell is present, it should be addressed. Its presence narrows the infundibulum and may be a contributing factor in the pathophysiology of recurrent acute sinusitis.
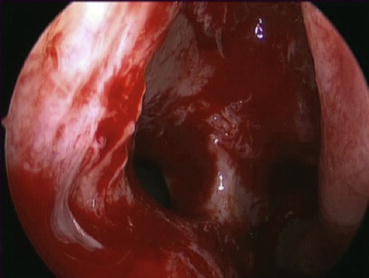

Fig. 22.2
Maxillary antrostomy
The optimal size of the antrostomy itself is still a matter of debate. Proponents of a small opening state that a larger ostium may have a drying effect of the sinus mucosa and that there may be a decrease in nitric oxide (NO) concentration after surgery. The clinical importance of such a reduction in NO level is still not well defined as evidenced by a recent meta-analysis [40]. On the other hand, a large antrostomy may lessen the risk of restenosis and improve access to topical therapies and cultures in the postoperative period.
Sphenoethmoidectomy
The next step is the performance of an anterior ethmoidectomy. The ethmoid bulla is the largest of the ethmoidal cells and the most prominent structure visualized when the uncinate is removed. With the use of a 0° scope, the middle turbinate is once again gently medialized in order to visualize the cleft between the bulla and the middle turbinate, the superior hiatus semilunaris. A curette is slid in that space and then more posterolaterally if a retrobullar recess exists. The bulla as a whole can be brought forward with the curette making this approach very safe for the orbit (Fig. 22.3a, b).
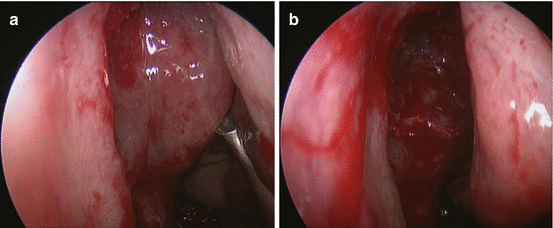

Fig. 22.3
(a) Before anterior ethmoidectomy. (b) After anterior ethmoidectomy
The remaining mucosa and bony lamellae are cleared to expose the basal lamella of the middle turbinate and to begin skeletonizing the lamina papyracea. The basal lamella often is not smooth but rather has indentations from ethmoid cells. Entry into the posterior ethmoid cells is through this lamella in an inferior and medial position immediately superior to the horizontal portion of the middle turbinate. This avoids injury to the middle turbinate branch of the sphenopalatine artery. The same low and medial approach is also used in the posterior ethmoid region to avoid injury to the lamina papyracea and the skull base until they can be definitively identified later in the procedure. The basal lamella of the superior turbinate is then traversed to bring the dissection into the sphenoethmoidal recess. At this point, the superior turbinate can be identified, and its inferior third is resected using through-cutting instruments in order to preserve the olfactory mucosa. Next, the natural sphenoid ostium is identified and opened to a diameter of at least 5–6 mm while preventing circumferential damage to the mucosa, which could lead to future ostium stenosis. A circular mushroom punch is useful for this maneuver.
An alternative way of entering the sphenoid has been described by Bolger using the parallelogram-shaped box [41]. The borders are the medial orbital wall laterally, the skull base superiorly, the superior turbinate medially, and the basal lamella of the superior turbinate inferiorly. The sphenoidotomy should be safe if performed low and medial in that box. However, this could be problematic if the superior turbinate attaches very laterally on the sphenoid face because this could potentially put the posterior orbit, optic nerve, or internal carotid injury at risk [42].
Following the sphenoidotomy, the skull base can be identified in the sphenoid sinus. Completing the ethmoidectomy entails removal of all the bony partitions comprising the posterior ethmoids, allowing for skeletonization of the skull base and lamina papyracea. Cells are often left undissected posteriorly close to the orbital wall since the orbit is cone shaped and the ethmoid cavity is larger posteriorly than anteriorly. Probing behind these lamellae and then curetting them or removing them with through-cutting instruments will ensure complete marsupialization of the ethmoid cavity (Fig. 22.4).
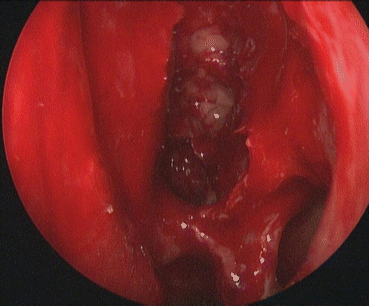

Fig. 22.4
Complete left sphenoethmoidectomy cavity
The complete removal of the bony lamellae is imperative, as residual partition may thicken causing ongoing inflammation and chronic osteitis. The same process may also happen if mucosal stripping occurs. Exposure of the bony surfaces can lead to mucosal metaplasia and non-physiological healing.
Frontal Recess Surgery
The extent of frontal sinus surgery should be individualized based on the extent of disease, frontal recess anatomy, and associated patient symptomatology.
The surgical anatomy of the frontal recess represents the most complex region of the sinonasal anatomy. It is an inverted funnel-shaped space from the frontal ostium to the ethmoid infundibulum or middle meatus, depending on the superior attachment of the uncinate process. It is bordered by the anterior attachment of the middle turbinate medially and by the medial orbital wall laterally. A sagittal view of the frontal recess is key to understanding its relationship with the skull base and the ethmoid bulla posteriorly and the agger nasi cell and frontal beak anteriorly. The goal of frontal recess surgery is to remove all the bony partitions that are present within these previously described limits in order to create wide access to the frontal sinus (Fig. 22.5).
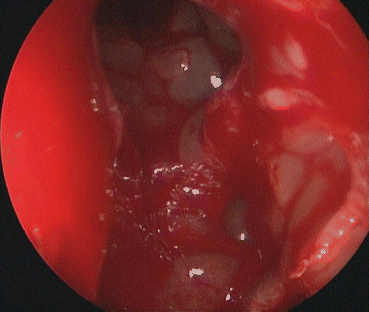

Fig. 22.5
Left frontal recess dissection
CT imaging is also essential to understanding the various cells that occupy the frontal recess. Agger nasi cell, the most constant anterior cell, is present over 95 % of the time [43]. Kuhn described four different types of frontal cells that can pneumatize over the agger nasi cell potentially narrowing the recess anteriorly. His classification system has been further revised by Wormald and Chan [44]. Type 1 cell describes 1 cell above the agger nasi cell, while type 2 denotes 2 or more cells above the agger nasi cell. Type 3 and 4 cells signify pneumatization above the frontal beak under 50 % of the sinus height or 50 % above the sinus height, respectively. These frontal cells have the potential to be large enough to be mistaken for the frontal sinus itself during surgery. Supraorbital cells are also a common finding. Visualization of a vertical septation in the frontal sinus on the preoperative CT scan is often correlated with the presence of these cells [45]. The supraorbital cell opening is posterior and lateral to the frontal sinus proper. Ideally, both these openings should be connected by removal of the intervening septation to prevent future stenosis.
Endoscopic frontal sinus surgery ensues with the use of angled scopes (30°, 45°, and 70°) and appropriately curved instruments. Navigating instruments are also helpful since the dissection will proceed in the vicinity of the skull base and orbit. Surgical navigation is also helpful for corroborating the surgeons’ impression of location of the recess and the intervening cells. Removing the most superior portion of the uncinate process and reshaping/widening the axilla of the middle turbinate are helpful steps at the beginning of the frontal sinusotomy, as these structures have the potential to obstruct optimal view.
The skull base is identified just posterior to the recess, and the bony partitions found anteriorly are brought forward with a 90° curved curette. A small tip navigating frontal seeker is then used to probe the frontal recess. Often this can be done between the medial wall of the agger nasi cell and the middle turbinate. The importance of completely “uncapping the egg” cannot be overstated as advocated by Stammberger. If these partitions are left undissected, the sinus will not drain adequately and potentially lead to scarring in the frontal recess. Preservation of the mucosa is also critical to prevent stenosis in these narrow confines. The role of stenting in the postoperative period is controversial, but it is generally reserved for revision and complex cases rather than primary surgeries.
Management of the Middle Turbinate
Ideal management of the middle turbinate signifies another important area of controversy. Two areas of thought have emerged since the beginning of ESS. Proponents of middle turbinate preservation argue that this leads to protection of the sinuses from inhaled irritants and maintenance of surgical landmarks for future surgeries and leaves intact the structures that are not affected by the disease process. Others have proposed routine turbinectomy while performing ESS especially in cases of nasal polyposis. Bulky middle turbinates because of either polypoid degeneration or pneumatization can contribute to OMC obstruction. Also, a “floppy” middle turbinate may have a tendency to scar to the lateral wall, compromising the delivery of topical therapies in the postoperative period. Despite the retrospective and prospective data demonstrating potentially better outcomes with partial turbinectomy, none of these studies was randomized thus introducing a major bias in the interpretation of the results [46, 47]. Consequently, there is still no definitive answer to the question of whether or not to resect all or a portion of the middle turbinate.
If the decision is to preserve the middle turbinate, then, measures to prevent lateralization are important. Multiple techniques and devices have been used with variable success. “Bolgerization” has been described which consists of creating a controlled synechiae between the medial aspect of the middle turbinate and the corresponding nasal septum, which would serve to maintain the turbinate in a medialized position [48]. Transseptal sutures holding both middle turbinates against the septum have also been described but are technically more difficult to perform. The use of middle meatal stents is often used, though options abound, ranging from resorbable to non-resorbable materials. A randomized controlled trial by Côté and Wright in 2010 established the efficacy of an absorbable dressing for the prevention of middle turbinate synechiae against the lateral nasal wall. The possibility of impregnating this dressing with a steroid solution had the added benefit of improved healing of the sinonasal cavities up to 6 months after surgery [49].
Conclusion
Over the past 30 years, there has been considerable evolution of the surgical paradigm in endoscopic sinus surgery. The main goal of surgery has changed from a strictly anatomically oriented approach to a wider marsupialization of the sinus cavities in order to optimize the delivery of topical nasal therapies in the postoperative period. Multiple technologies are also available to the otolaryngologist to enhance the safety and completeness of surgery, though intimate understanding of the anatomy and mucosal preservation technique remain key prerequisites to sinus surgery.
References
1.
Kennedy DW, Zinreich SJ, Rosenbaum AE, Johns ME. Functional endoscopic sinus surgery. Theory and diagnostic evaluation. Arch Otolaryngol (Chicago: 1960). 1985;111(9):576–82.







