Chapter 39 Electrical injury
Reconstructive problems
![]() Access the complete reference list online at http://www.expertconsult.com
Access the complete reference list online at http://www.expertconsult.com
Introduction
Although the incidence of low-voltage burns has declined steadily over recent decades, most probably due to progress made in the field of home and occupational safety education and equipment, electrical injuries still account for 3–5% of all admissions to major burn centers.1,2 Electrical fatalities are relatively uncommon and most of them occur accidentally. Earlier reported limb amputation rates of up to 71% decreased over recent decades with the increasing ability to reconstruct anatomic parts and restore function, but limb salvage remains a surgical challenge.3,4
Physiological basis of tissue destruction
The traditional pathophysiological understanding of electric injury was based on the assumption that the passage of electric current produces heat and triggers tissue damage.5 Thus, tissue-specific susceptibility (and vulnerability) is considered to increase progressively from nerve to blood vessels, muscle, skin, tendon, and fat to bone. As osseous tissue shows the highest electrical resistance it will generate the most heat. Electric current will preferentially take the path of least resistance through the body, so that the current will pass particularly along the neurovascular bundles. This theory further postulated that the lesions produced by the current would result in delayed vascular occlusion and progressive tissue necrosis.6
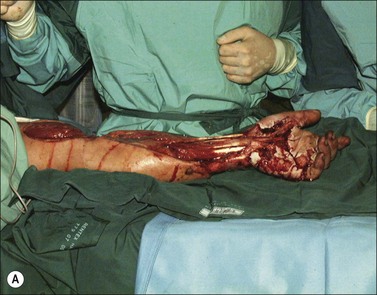
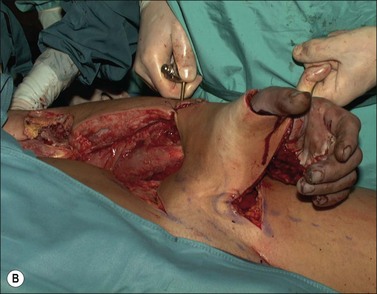
In high-voltage injuries the internal milieu acts as a single uniform resistance.7 Instead of conduction through specific preferential tissues, the body conducts the current with a composite resistance of all tissue components.8 The crucial factor in determining the resistance and hence the magnitude of tissue damage is the cross-sectional diameter of the affected body part. Devastating injuries to the extremities occur with a significantly higher frequency than tissue damage to the thorax and abdomen9 (Fig. 39.1). As muscle tissue occupies the largest cross-sectional area in the limb it also carries the predominant electric current. As joint areas are regions where the cross-sectional tissue composition changes from low-resistance muscle to high-resistance bone, tendon and skin, a proportionally higher current and heat are produced in these areas according to Ohm’s Law.9
Arcing describes the energy transmitted by a hot electrically conducting gas. However, this requires a voltage of more than 20 000 V to bridge even a short distance of 1 cm.10 Effects on tissue vary from minimal skin wounds to charring and tissue vaporization.
Electrical damage to a large artery represents a grave prognostic sign for limb survival.11 The reported risk of amputation is high, between 37% and 65%.12–14
Further non-thermal mechanisms of cellular injury have been defined. This includes a voltage-induced loss of cell membrane semipermeability.15–17 When the integrity of the cell membrane is lost, the impedance is markedly reduced, leading to a simultaneous increase in the area exposed to current flow.18 In addition to breakdown in cellular integrity electrical fields also induce denaturation of membrane proteins by altering their structural conformation and rendering them non-functional.19 Both mechanisms are responsible for rhabdomyolysis and secondary myoglobin release, which depends directly on the imposed electric current and is not a thermal effect.20
The human body consists of about 60 % water. The intra- and extracellular water content and their highly resistive plasma membrane separate these two compartments from each other.21 Change in the current transporters from electrons to ions at the skin surface appears as metallic condensations as dark-greyish skin lesions which resemble eschar.22
Diagnosis and acute treatment
Diagnosis and acute treatment of electrical injury are described in Chapter 38.
Assessment of tissue damage
Accurate assessment of the extent of tissue damage is difficult. The percentage of burned body surface area grossly underestimates the injury to underlying tissue. Electrical burns may appear as mere pinpoint marks. In contrast, fatal electrocution may even take place without visible skin burns in case of a large contact area (Table 39.1).
Table 39.1 Dependence of cutaneous resistance on skin moisture
| Resistance | |
|---|---|
| Dry skin | 100 000 Ω |
| Wet skin | 2 500 Ω |
| Skin immersion | 1 500 Ω |
| Rubber soles | 70 000 Ω |
Reproduced from Ohashi et al. Burns 1998;24:362–368.24
In contrast to thermal burns, deposition of metallic iron and copper is found on the epidermis after electrical injuries as electrolysis occurs in the extra cellular fluid of the skin.24–26
Clinical determination of tissue viability is based on inspection and the demonstration of muscle contractility. As yet, there are no other diagnostic tools available to accurately assess the extent of tissue damage in the early phase following electrical injuries. The value of magnetic resonance imaging (MRI) for the detection of non-perfused non-edematous muscle is debated.24–26 Angiography, although not providing information on tissue viability, demonstrates the absence of tissue perfusion and may lead to an early indication for limb amputation.27
Rhabdomyolysis and myoglobinuria
Destroyed muscle cells release myoglobin, resulting in myoglobinemia. Hemolysis also often occurs with electrical injury. Serum levels of creatinine and creatinine phosphokinase (CPK) are used as indicators of rhabdomyolysis. After muscle injury CPK levels will peak by 24 hours and return to baseline within 48–72 hours.28 This diminishes the diagnostic value of serum and urine chemistry testing.29,30
Renal failure
Myoglobinuria has traditionally been considered a major risk factor for the development of acute renal failure. Recently, patients with electrical injuries have been shown to have a surprisingly low risk for renal failure.31 In 162 patients, only 14% had myoglobinuria and none developed renal failure. Suggested criteria to evaluate the risk of acute renal failure after electrical injury include prehospital cardiac arrest, full-thickness burns, compartment syndrome, and high-voltage injury. The presence of at least two of these criteria should instigate immediate treatment, as the timeframe to prevent progression to acute renal failure is limited to a few hours post injury.
Cardiac monitoring
Among the estimated 1300 deaths that occur annually in the United States from electrical injury (including lightning strike), 30% of patients present with cardiac complications. The majority of ECG abnormalities are sinus tachycardia and non-specific changes in the ST-segment and T-wave.32 Death from electric injury most commonly results from current-induced cardiac arrest.
However, if an initial ECG shows no abnormalities the delayed development of cardiac problems is very unlikely, irrespective of whether the patient sustained high- or low-voltage injuries.33–35
Conversely, the presence of dysrhythmias or conduction abnormalities on initial presentation, electrical injury in children, or the projected path of the current flow crossing the thorax on initial presentation, warrant prolonged cardiac monitoring.36–39 Depending on the nature of the current-induced arrhythmia, pharmacotherapeutic and/or invasive interventions may be necessary. Table 39.2 outlines recommendations for cardiac monitoring of electrically injured patients based on central clinical findings and specific features of the trauma mechanism.42
Table 39.2 Recommendation for cardiac monitoring in the absence of other injuries
| Admission and cardiac monitoring | Discharge |
|---|---|
| Loss of consciousness59 | Asymptomatic patient60 |
| Extensive burns50 | Normal initial ECG61 |
| Current passing through the thorax52 | Uneventful 4-hour observation62 |
| Cardiac dysrhythmias59 | Voltage less than 240/260 in adults60,61 Voltage less than 120/240 in children48,62 PP. 1257–1259 |
Surgical debridement
In recent decades the concept of progressive tissue necrosis has led to the treatment strategy of early debridement and fasciotomy, followed by serial debridement and delayed wound closure.41 The observed changes may likely be explained by vascular changes similar to ischemia–reperfusion injury with immediate cessation of capillary blood flow in response to current passage. This event is followed by vascular spasms lasting for an extended period, with subsequent vasodilatation and restoration of flow.43–45 Although still controversial, these findings may alter the acute treatment paradigm.
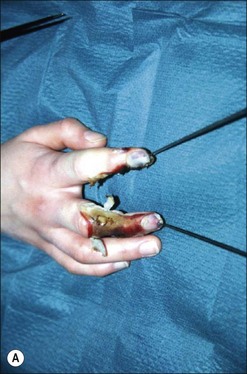
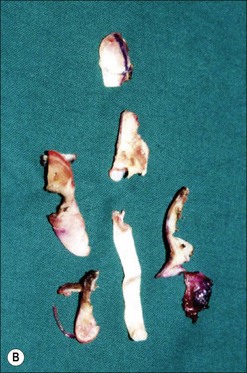
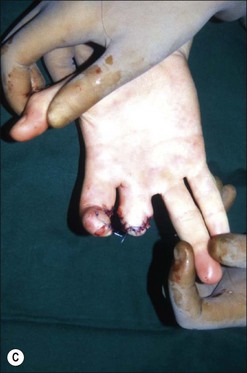
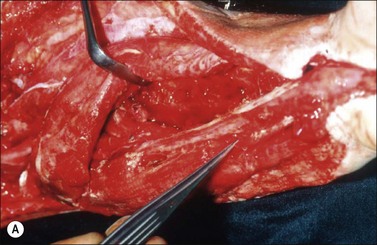
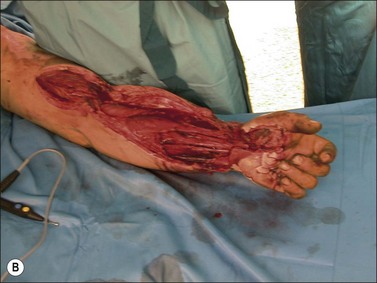
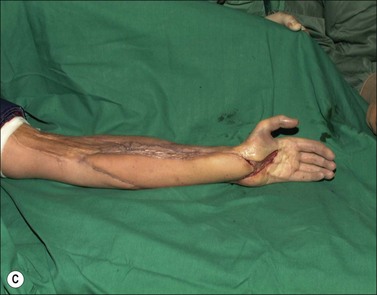
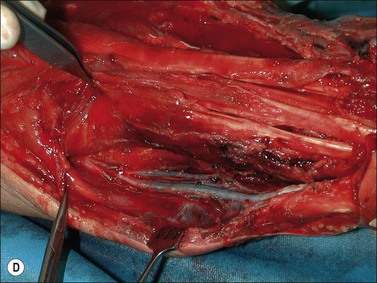
Although there is little discussion about the early time point of debridement, several recent studies question the need for extensive and total necrectomy and advocate the approach of delayed soft tissue coverage. ‘Conservative debridement’ consisting of removal of charred and obviously necrotic tissue was promoted in a study on 40 patients.46 In this study partially damaged tendons, muscles, and nerves were preserved, and wound closure was achieved by immediate flap coverage. Patients treated in this manner with immediate soft tissue coverage had a significantly better outcome than a control group who underwent serial debridement procedures. Similar results were found in a study using early free-flap coverage for electrical injuries, suggesting that careful limited initial debridement is an adequate measure.47,48 According to this study overly extensive and repeated debridement to ensure viability of all remaining tissue appears not only unnecessary but quite likely harmful. It appears safe to abandon these strategies and to perform an early, extensive but selective debridement in order to preserve continuity of functionally important structures (Fig. 39.2). Limb salvage with functional preservation of vital structures should be attempted and may require revascularization using segmental vein grafts or segmental cable grafting of nerves. Pedicled flaps should be considered in cases of suspected arterial compromise.
Despite the encouraging results of the studies recommending early soft tissue reconstruction, by all means it should be noted that for the extent of electrical injury no scoring system has so far been established. Alternative approaches to salvage upper extremity function, such as the temporary ectopic implantation of an undamaged hand, have been reported but cannot be considered standard of care.49
Compartment syndrome
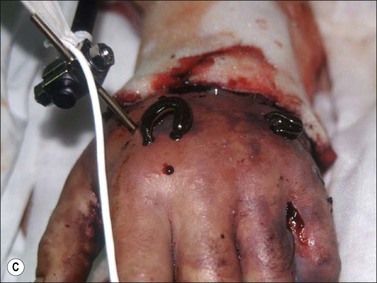
Muscle compartment pressure should be monitored clinically and by invasive pressure measurements. In contrast to blunt trauma, pain is not a reliable indicator of increased compartment pressure owing to the high incidence of electrical nerve injury. When compartment pressures exceed 30 mmHg surgical decompression by open fasciotomy becomes necessary to prevent ischemic muscle injury. In case of lower compartment pressure, progression may be prevented by administration of non-steroidal anti-inflammatory drugs (NSAIDs) and antioxidants, protective splinting, and rest without elevation of the affected extremities. However, general operative decompression in high-voltage injuries to extremities appears not to be warranted. In a cohort study, Mann et al. found an increased amputation rate of 45% with immediate operative decompression compared to patients undergoing selective fasciotomy, and they recommend fasciotomy only in cases of progressive peripheral nerve dysfunction, manifested compartment syndrome, or other major injuries40 (Fig. 39.2 and Fig. 39.3) With a fixed neurological deficit, however, surgical decompression shows no influence on outcome.50 If the clinical situation remains doubtful, however, the performance of open fasciotomy with early debridement may be preferred.
Head: scalp, skull and mouth
Exposure and necrosis of osseous structures may lead to osteomyelitis and epidural abscess formation. Treatment strategies depend on the extent of the injury to the bone. In case of only a partial necrosis of the bone the outer table of the skull can be tangentially removed and the viable diploic cavity exposed. In cases of sufficient vascularization the exposed bone can be grafted immediately or, when blood supply is questionable, grafted when suitable granulation tissue has developed.51 Vacuum sealing of the wound can be of significant efficacy in growing adequate amounts of granulation tissue. When the initial wound debridement is delayed, necrotic and infected bone potentially becomes the source of a full-thickness skull defect. Full-thickness injury of the skull theoretically requires complete excision of the necrotic bone to prevent infectious complications.69 Another approach suggested is partial debridement followed by definitive flap coverage of the exposed bone. This, however, requires early debridement and the prevention of localized bacterial colonization and infection.52,53
In a series of 10 male patients with non-viable cranial bone after class IV electrical burns, 3 weeks after initial soft-tissue debridement multiple burr holes in the non-viable and preserved bone were made and scalp flap coverage was performed.54 Leaving the necrotic osseous structures in situ serves as a scaffold of substitution for bone regeneration.55–57
Skull contour was maintained in all 10 patients, making a secondary cranioplasty unnecessary. During a follow-up period of at least 1 year no postoperative infection, osteomyelitis, or cranial bone sequestration occurred.54
Another option is the use of glycerol-preserved allografts (GPA).58
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree