Figure 25-1 Herpes simplex. A: Recurrent HSV-1 infection showing grouped vesicles on an erythematous base, occurring most commonly on or near the vermilion border of the lips. B: Herpes simplex. HSV-2 infection showing grouped erosions and a few vesicles on the genital area.
Eczema herpeticum is a potentially life-threatening herpetic infection of a preexisting skin disease (10). It occurs most commonly in patients with atopic eczema; however, it may also occur in other skin diseases such as seborrheic dermatitis, pityriasis rubra pilaris, pemphigus foliaceus, Darier disease, and post–perioral dermabrasion (11). An extensive rash with small vesicles filled with yellow pus and high fever is the common presentation of eczema herpeticum (Fig. 25-2), which has been linked to increased interleukin (IL) 4 and decreased natural killer cells and IL-2 receptors in patients with atopic eczema (12,13). Herpetic folliculitis presents as painful, grouped erythematous, perifollicular vesicles that do not respond to antibacterial or antifungal agents, usually occurring in the bearded region and scalp (14). The characteristic vesiculofollicular lesions usually heal within a few weeks. Herpetic whitlow manifests as painful, deep-seated vesicles limited to the paronychial or volar aspects of the distal phalanx of a finger. Herpetic whitlow occurs largely in medical and dental personnel following minor injuries and may be caused by either HSV-1 or HSV-2 (15). Other forms of HSV infections include HSV keratoconjunctivitis, which is the number one cause of corneal blindness in the United States (16); and a rare necrotizing balanitis (17). Noncutaneous forms of herpes simplex infection include herpetic oropharyngitis, pneumonia, encephalitis, esophagitis, and proctitis.
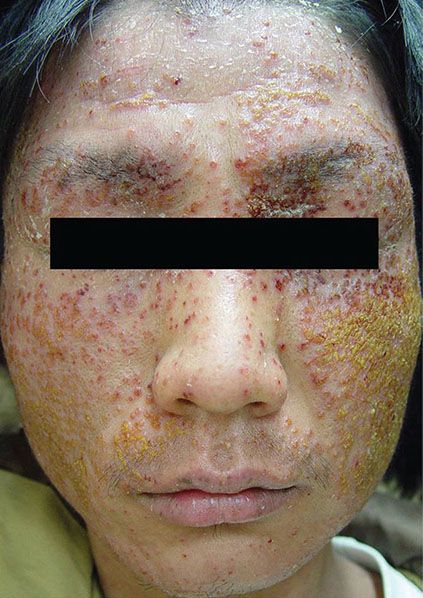
Figure 25-2 Eczema herpeticum. Extensive vesicles and crusts on the face. This lesion often occurs in atopic patients.
Herpes Simplex in Immunocompromised Hosts
Severe primary or secondary herpetic infection with systemic involvement may occur in immunocompromised patients. Coinfection with HSV and HIV frequently occurs, probably because they potentiate each other’s transmission. About 70% of HIV-positive patients are seropositive for HSV-2 (18). Three forms of herpes simplex are characteristically found in children or adults with impairment of the cellular immune system. The most common of the three forms is chronic ulcerative herpes simplex, and the other two are generalized acute mucocutaneous herpes simplex and systemic herpes simplex.
Chronic ulcerative herpes simplex exhibits persistent ulcers and erosions, usually starting on the face or in the perineal region (19). Without treatment, gradual widespread extension is the rule. Some patients may develop chronic verrucous changes within and around the persistent ulcer, particularly in the presence of antiviral-resistant herpes infections. The infection may progress into systemic herpes simplex. Generalized acute mucocutaneous herpes simplex follows an initial localized vesicular eruption. Dissemination associated with fever takes place, suggestive of smallpox or varicella infection (20). Systemic herpes simplex usually follows oral or genital lesions of herpes simplex (Fig. 25-3). Areas of necrosis, particularly in the liver, adrenals, pancreas, and brain, lead rapidly to death. In some patients, a few cutaneous lesions of herpes simplex also exist.
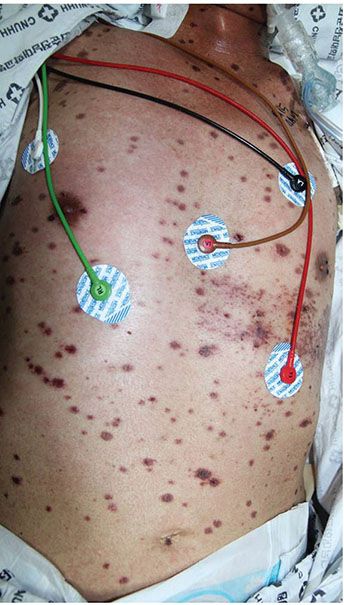
Figure 25-3 Generalized herpes simplex. In an immunocompromised patient, herpes simplex infection can be generalized manifesting as scattered necrotic erosive vesicles on the entire body.
Congenital Herpes Simplex
Because almost 1% of patients in prenatal clinics have HSV-2 infections by culture of the vaginal cells and one third of these have active lesions, there exists the potential of congenital herpes simplex infection. It is important, however, to distinguish primary HSV-2 infection of the mother from recurrent infection. In recurrent infection, the presence of neutralizing antibodies to HSV-2 results in a low rate of attack of the fetus (21). In maternal HSV-2 primary infection, the mode of infection of the infant is important. Transplacental infection of the fetus occurring during the first 8 weeks of gestation produces severe congenital malformations. If infection occurs at a later time, malformations are less severe and consist of growth and psychomotor retardation, microcephaly, and a widespread, recurrent vesicular eruption that can mimic a mechanobullous disorder.
Localized herpetic lesions several days after delivery are the initial manifestation if infection is acquired in the birth canal. In vertex deliveries, the scalp is a common site for the development of initial herpetic vesicles. It is rare for transplacental or neonatal infection to remain limited to the skin. It can be followed by systemic herpetic infection, such as encephalitis, hepatoadrenal necrosis, and pneumonia (7).
Herpes Simplex Associated with Erythema Multiforme
HSV is the most commonly identified etiologic agent in recurrent erythema multiforme (22). Molecular diagnostic methods, primarily the PCR, have been used to detect herpetic DNA in the skin lesions of recurrent erythema multiforme in both adults and children (23). The association is further supported by suppression of recurrent lesions in this group by therapy directed at HSV. These patients present clinically with herpetic outbreaks and target lesions symmetrically on acral sites, distant from the herpetic lesions themselves.
Histopathology of Herpes Simplex
The earliest changes of herpes simplex lesions include nuclear swelling of keratinocytes. With hematoxylin and eosin stains, these nuclei appear slate gray and homogeneous with margination of the nuclear chromatin (Fig. 25-4A). A few necrotic keratinocytes in the epidermis may also be seen. The changes usually begin along the basal layer keratinocytes and then involve the entire epidermis. Later, skin herpes simplex produces profound degeneration of keratinocytes, resulting in acantholysis and vesicle formation (Fig. 25-4B). Degeneration of epidermal keratinocytes occurs in two forms: ballooning degeneration and reticular degeneration. Ballooning degeneration is swelling of epidermal keratinocytes. Balloon cells have a homogeneous, eosinophilic cytoplasm. They may be multinucleated (Tzanck cells). Balloon cells lose their intercellular bridges and become separated from one another (secondary acantholysis), and unilocular vesicles result. Ballooning degeneration occurs mainly at the bases of viral vesicles, so that the vesicle formed intraepidermally ultimately may be subepidermal. Ballooning degeneration can affect epithelial cells of hair follicles, sebaceous glands, and rarely eccrine duct cells (Fig. 25-4B). Reticular degeneration is the result of rupture of ballooning cells due to progressive hydropic swelling of the epidermal cells. Reticular degeneration occurs in the upper portions and at the peripheries of viral vesicles. Reticular degeneration is not specific for viral vesicles, because it also occurs in the vesicles of contact dermatitis. Through coalescence of cells with reticular degeneration, a multilocular vesicle may result, the septa of which are formed by residual cell membrane. In older vesicles, the cellular walls disappear. The originally multilocular portions of the vesicle may become unilocular.
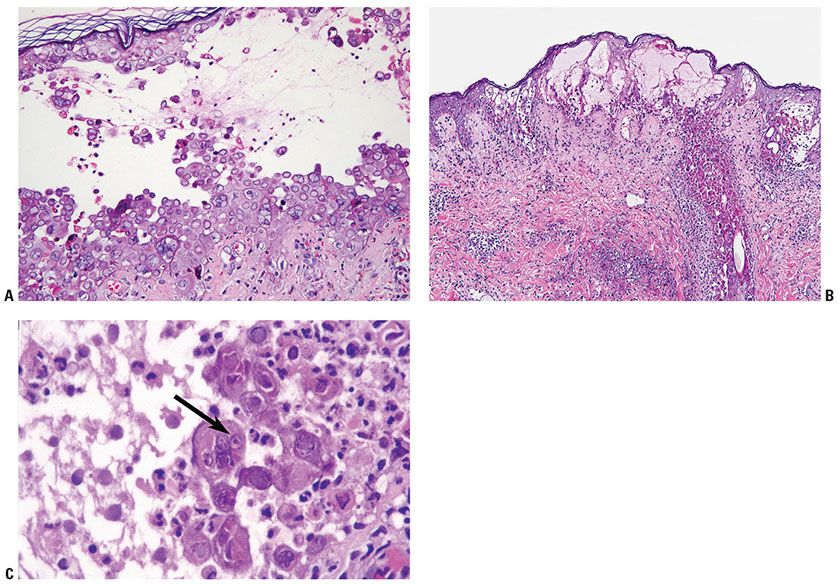
Figure 25-4 Herpes simplex. A: On high magnification, balloon cells at the floor of a vesicle are shown; these cells exhibit characteristic ballooning degeneration with steel gray nuclei with homogeneous pale chromatin and nuclei molding. B: An intraepidermal vesicular lesion with marked acantholysis and reticular degeneration of the cells at the epidermis. There are many necrotic cells in the follicular epithelium (low magnification). C: Balloon cells at the floor of a vesicle are shown. In the center, eosinophilic inclusion bodies surrounded by a halo lies in the nuclei of balloon cells (arrow points to the intranuclear inclusion). Other cells exhibit the more characteristic pattern of homogeneous pale chromatin without inclusion bodies.
Inclusion bodies may be observed in the centers of enlarged, round nuclei of balloon cells. The inclusion bodies are eosinophilic and surrounded by a clear space or halo (Fig. 25-4C). They measure from 3 to 8 μm in diameter. The majority of the balloon cells at the floor of a vesicle exhibit characteristic homogeneous pale chromatin and nuclei molding without inclusion bodies (Fig. 25-4A). Neutrophils are often present within established vesicles. Neutrophils may be prominent in the lesions of herpetic whitlow. The upper dermis beneath viral vesicles contains an inflammatory infiltrate of variable density. In some cases of herpes simplex, vascular damage is present, showing necrosis of vessel walls, microthrombi, and hemorrhage subjacent to the vesicle (Fig. 25-5B). In addition, eosinophilic inclusions may be found in endothelial cells and fibroblasts. In later lesions, necrosis of epidermis is often present with abundant neutrophils (Fig. 25-5A). A dense mixed cell infiltrate in the upper dermis with ghosts of necrotic multinucleated acantholytic cells with slate gray nuclei may still be seen. As in all vesiculobullous diseases, an early lesion should be selected for biopsy; otherwise, secondary changes, especially invasion of inflammatory cells, may obscure the diagnostic features.
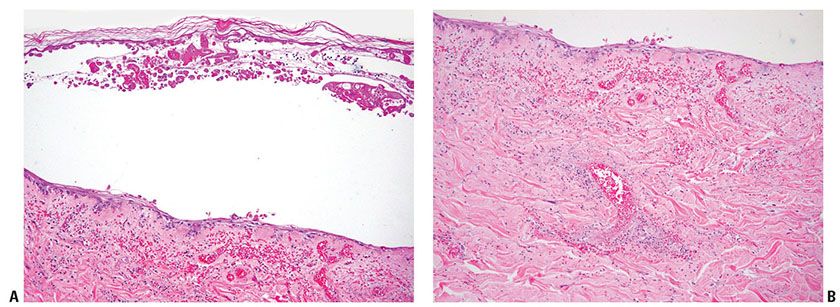
Figure 25-5 Herpes simplex (biopsy from the patient of Fig. 25-3). A: There are marked epidermal necrotic cells appearing as ghost cells and hemorrhages and some inflammatory cells in the dermis at the later stage of herpes simplex infection. B: In the dermis, marked hemorrhages and vascular damages are seen, revealing fibrinoid necrosis on the vessel walls and prominent nuclear dust with many inflammatory cells around vessels.
Herpetic involvement of hair follicle and pilosebaceous units is not uncommon in recurrent lesions (24). In fact necrosis of a pilosebaceous unit is a clue to search for multinucleated cells with characteristic slate gray nuclei. In herpetic folliculitis, pilosebaceous involvement is a predominant feature. Rarely, the eccrine ducts and glands are involved by the virus. The histology of eczema herpeticum is similar to that of vesicles of herpes simplex, but usually with more dense neutrophilic infiltrates.
Chronic ulcerative herpes simplex may be difficult to recognize as being of viral genesis if the epidermis is absent. However, viral cytopathic changes may be observed in keratinocytes at the margin of the ulcer. Viral cultures or typing of HSV-1 and HSV-2 by direct immunofluorescence or immunoperoxidase technique can be used to prove the diagnosis. However, the most rapid and accurate method may be the use of molecular diagnostic methods such as the PCR (25).
Tzanck Smear. Cytologic examination of a Giemsa-stained smear taken from the floor of a freshly opened early vesicle is often useful. The presence of many balloon cells results from the fact that the smear is taken from the floor of the blister, where ballooning degeneration is most pronounced (Fig. 25-6). Many acantholytic balloon cells with one or several nuclei may be seen in herpes simplex, varicella, and zoster. The Tzanck smear cannot distinguish among HSV-1, HSV-2, and VZV.
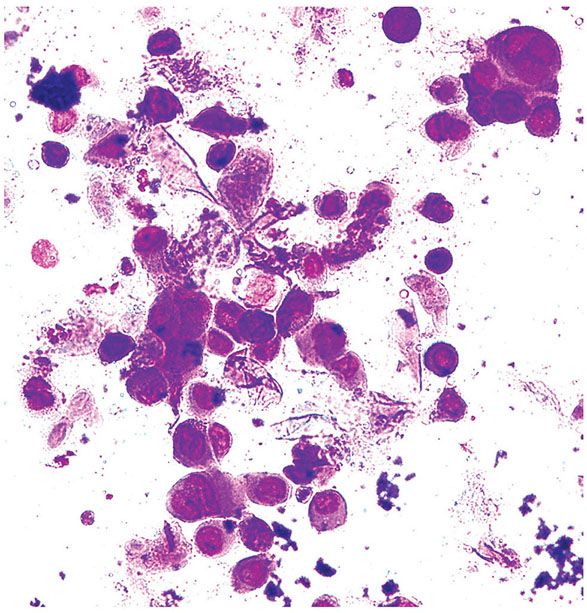
Figure 25-6 Tzanck smear. A Tzanck smear from the floor of the vesicle of herpes simplex shows multinucleated giant cells and ballooning cells. There is also chromatin margination in the nuclei of infected cells (Wright–Giemsa stain).
Histogenesis and Viral Identification. The HSV can be directly identified by culture, direct immunofluorescence testing, in situ hybridization, immunoperoxidase staining, or molecular diagnostic tests. The gold standard for diagnosis is a viral culture. For culture, material from the floor of a blister or other infected material is inoculated onto HeLa cells, human amnion cells, or fibroblasts. The virus has cytopathic effects on the cultured cells. Direct immunofluorescence examination for the presence of the viral antigen in cells infected with HSV is then performed. Herpes simplex DNA can be extracted and amplified by PCR from stained or unstained Tzanck smears, crusts, fresh tissue, and paraffin sections of suspected lesions. With appropriate primers, each herpes-type virus can be specifically identified. Viral culture is particularly important in cases of chronic infection refractory to therapy, as viral culture is currently the gold standard for identifying resistance to antiviral medications.
For distinction between the two types of HSV, immunoperoxidase staining may be performed on sections of the lesions using monoclonal antibodies, one directed against HSV-1 and the other against HSV-2. In situ hybridization can also be used for visual demonstration of viral genomic material in tissue (26). For determination of the antibody titer in the patient’s serum, a complement fixation reaction is usually used. The serologic test for HSV is useful to confirm HSV infection in persons with a questionable history of herpetic disease and in those who have unrecognized or subclinical infection so that they may be aware of their potential to transmit the virus.
On electron microscopy, the HSV is spherical. Its DNA-containing core measures approximately 40 nm in diameter. The virion has a diameter of about 100 nm and, together with its outer coat, measures around 135 nm (27). Ultrastructurally, the virion of herpes simplex is indistinguishable from that of varicella.
Differential Diagnosis. Viral vesicles produced by the HSV and by the VZV are identical. Furthermore, an incompletely developed case of herpes zoster can mimic herpes simplex infection, both clinically and histologically. In the context of immunosuppression, chronic cutaneous forms of both viruses exist and their specific identification may not be possible by clinical morphology or light microscopy. Molecular diagnostic methods are the quickest and most reliable way to separate and identify the specific viruses in these situations.
Kaposi Varicelliform Eruption
Clinical Summary. Kaposi varicelliform eruption is the name given to a distinct cutaneous eruption caused by HSV-1 and HSV-2, vaccinia virus, and Coxsackie A16 virus, superimposed on a preexisting dermatosis (28), usually atopic dermatitis (29). Occasionally, seborrheic dermatitis or other dermatoses, such as Darier disease, benign familial pemphigus, pemphigus foliaceus, mycosis fungoides, Sézary syndrome, or ichthyosis vulgaris, provide the “soil” in which Kaposi varicelliform eruption develops (11).
Eczema herpeticum is usually caused by HSV-1 and, on rare occasions, by HSV-2. Eczema herpeticum can occur as either a primary or a recurrent type of infection. A primary infection with HSV-1 occurs in persons without circulating HSV-1 antibodies. The great majority of patients with the primary type of eczema herpeticum are infants and children. The recurrent type of eczema herpeticum occurs in patients with circulating HSV-1 antibodies. The first attack of eczema herpeticum, whether of the primary or the recurrent type, may be the result of exogenous infection, whereas subsequent attacks may result from either reinfection or reactivation. The primary type of eczema herpeticum can be a serious disease with viremia and potential internal organ involvement resulting in death. In contrast, the recurrent type of eczema herpeticum generally shows no viremia and internal organ involvement except in immunologically compromised patients. Occasionally, secondary bacterial infection with subsequent septicemia may cause death. The mortality associated with eczema herpeticum is approximately 10%, with most fatalities occurring in infants and children with a primary type of herpetic infection and in adults with inadequate cellular immunity.
Clinically, all forms of eczema herpeticum and eczema vaccinatum look alike, but eczema vaccinatum has not been seen in modern practice because vaccinia virus has not been used, until recently for smallpox prevention (30). Both show a more or less extensive eruption composed of vesicles and pustules that may be umbilicated. These vesicles and pustules occur chiefly in the areas of the preexisting dermatosis but also on normal skin. The face is usually severely affected and may be edematous. There may be fever and prostration.
Histopathology. Both eczema herpeticum and eczema vaccinatum show vesicles and pustules of the viral type. Even though the pustules exhibit only necrotic epidermis in their centers, one may still see reticular and ballooning degeneration at their peripheries. In eczema herpeticum, but not in eczema vaccinatum, multinucleated epithelial giant cells are often present.
Differential Diagnosis. Because eczema herpeticum and eczema vaccinatum look alike clinically, and the histologic similarity is great in the absence of inclusion bodies and of multinucleated epithelial cells, differentiation of the two diseases may have to depend on nonhistologic means, such as a history of possible exposure to the vaccinia virus. The most efficient and rapid means of identifying the viral agent is to use DNA amplification methods and appropriate probes.
Principles of Management. For all herpes simplex infections, the mainstay of treatment is antiviral medications. Generally, acyclovir, valacyclovir, or famciclovir is used as a first-line agent. Acyclovir is a nucleoside analog, a competitive inhibitor of the viral polymerase, and prematurely terminates viral DNA synthesis. Famciclovir and valaciclovir are prodrugs, and they are metabolized into active drugs in the body as penciclovir and acyclovir, respectively. Topical antiviral agents are used for HSV-1 and -2 infections. Immunosuppressed patients or those with widespread infection may benefit from parenteral administration. Once the vesicles develop and evolve into erosions, patients are at risk of secondary infection; meticulous skin care is essential to minimize scarring and prevent bacterial superinfection. Antiviral resistance may develop; second-line therapies include foscarnet, and in some cases, cidofovir may be used. Immunosuppressed patients with chronic, verrucous resistant HSV may require either local or systemic cidofovir to achieve complete clearance (31,32).
Varicella and Herpes Zoster
Varicella (chickenpox) and herpes zoster (shingles) are produced by the same virus, the VZV. VZV is endemic in the population but becomes epidemic during late winter and early spring (33). Varicella results from contact of a nonimmune person with this virus, whereas herpes zoster occurs in persons who have had previous varicella, either clinically or subclinically. Herpes zoster is caused by reactivation of a latent infection in either a spinal or a cranial sensory ganglion. On reactivation, the virus spreads from the ganglion along the corresponding sensory nerve or nerves to the skin.
Varicella
Clinical Summary. In varicella, a generalized eruption develops after an incubation period of about 2 weeks. Varicella is primarily a disease of childhood; 90% of cases occur in children aged 14 years or younger (34). The transmission rate of acute varicella to susceptible household contacts is estimated to be as high as 80% to 90%, making it one of the most infectious viral diseases of humans. VZV is acquired from patients with primary varicella or herpes zoster through either direct contact with infected vesicular fluid or inhalation of aerosolized respiratory secretions. Recent experiments suggest that the VZV is lymphotropic, especially to T cells. After the initial viremia and a period of viral replication, a second, higher-titer viremic phase results in widespread dissemination of the virus. The rash develops as VZV-infected mononuclear cells invade vascular endothelial cells, gaining access to cutaneous tissue. After the primary varicella infection resolves, the VZV enters a latent phase in the dorsal root ganglia (35). Pruritic rash of chickenpox, the hallmark of the disease, typically begins on the head and spreads to the trunk and finally the extremities; proximal involvement is greater than distal. The skin lesions of varicella begin as erythematous macules to small papules, which develop into vesicles (Fig. 25-7). In mild cases, most vesicles become crusted without changing into pustules. In severe cases, the vesicles may have slightly hemorrhagic bases (“dewdrops on rose petals”). New lesions continue to develop for several days, and so lesions in different stages, papules, vesicles, pustules, and crusted lesions can be observed. As childhood varicella vaccination is now accepted practice, occasionally brief, attenuated courses of disease may develop in previously vaccinated patients. Typically, varicella lesions heal without scarring as new epithelium forms at the base; however, hypopigmentation, skin pitting, and keloid formation may result, especially among darker-skinned individuals. Varicella in adults is generally associated with a greater number of skin lesions, more systemic complaints, and a higher risk of such serious complications as pneumonia, encephalitis, and death. Subclinical varicella, documented by increases in antibody titer after exposure to the virus, has been estimated to occur in as much as 5% of the population (36).
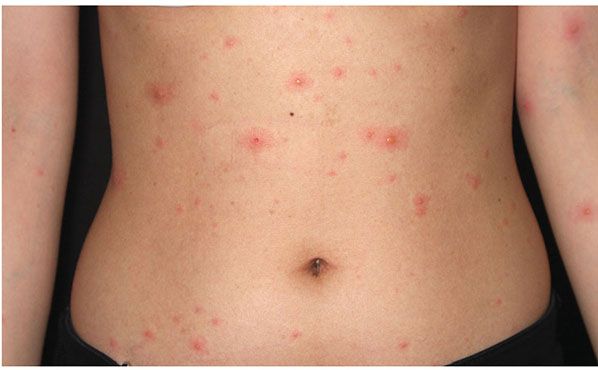
Figure 25-7 Varicella (chickenpox). A vesicular eruption in crops with a “dew drops on rose petals” appearance.
Three systemic complications can occur in varicella without the existence of immunosuppression: primary varicella pneumonia, Reye syndrome, and varicella of the fetus and newborn. Primary varicella pneumonia occurs in approximately 14% of adults with varicella and carries a significant mortality. Reye syndrome is an acute, severe, and usually fatal encephalopathy associated with fatty degeneration of the viscera, particularly of the liver. It follows mainly varicella but also other viral infections. The incidence of Reye syndrome declined soon after the report of the epidemiologic association of Reye syndrome and the ingestion of aspirin (37). Varicella in the first 20 weeks of pregnancy has a 2% risk of producing embryopathy or other congenital malformations (38). Maternal varicella late in pregnancy may be transmitted to the fetus transplacentally or may contaminate the baby during passage through the birth canal, resulting in neonatal varicella.
Varicella in Immunocompromised Hosts
In patients with impairment of the cellular immune system, continued viral replication may lead to a prolonged course and to dissemination to various organs, particularly the lungs and liver. Varicella pneumonia is not uncommon, even in children, and death may result from dissemination. VZV may produce particularly severe problems for patients with AIDS. There may be continuous dissemination of vesicular lesions, formation of chronic vegetative lesions, and large necrotic lesions (39).
Herpes Zoster
Clinical Summary. It is known that VZV establishes latency in ganglia following the primary infection causing varicella (chickenpox) and that the virus may reactivate after years of dormancy to produce herpes zoster (shingles). It is now widely accepted that the virus is mainly latent in neuronal cells, with only a small proportion of nonneuronal cells infected. The ganglia most often involved are those of the lumbar and thoracic nerves. When the virus is reactivated, newly synthesized viruses are transported along the sensory nerve and released into the skin. Trigger mechanisms include trauma, stress, old age, and immunosuppression. The incidence of herpes zoster (shingles) increases with age (40). Although herpes zoster occurs largely in adults, particularly in those of advanced age, about 5% of patients with herpes zoster are children less than 15 years of age. The course in children without immune defects usually is mild. The eruption in herpes zoster consists of grouped vesicles on inflammatory bases and arranged along the course of a sensory nerve (Fig. 25-8). The bases of the lesions frequently are hemorrhagic, and some may become necrotic and ulcerate. Not infrequently, there are a few scattered nondermatomal lesions, and rarely there is a generalized eruption, including mucosal lesions, indistinguishable from that of varicella. The most common places of involvement include the thoracolumbar (T3-L2) and facial (V1) dermatomes. Severe neuritis with acute pain, dysesthesia, and skin hypersensitivity is common and more severe in people older than 60 and in immunocompromised patients. Zoster sine herpete, or “pain without the rash,” is a rare presentation of herpes zoster; dermatomal pain is present with failure to develop a rash, likely because of neural inflammation and damage caused by viral reactivation. There exists a zoster vaccine that may offer some protection, approved for patients age 60 and older. Herpes zoster during pregnancy is not as serious a problem as primary varicella during pregnancy; it does not result in serious morbidity or intrauterine VZV infection. Transplacental transmission of the virus does not occur with maternal herpes zoster; preexisting normal immunity is thought to protect the fetus.
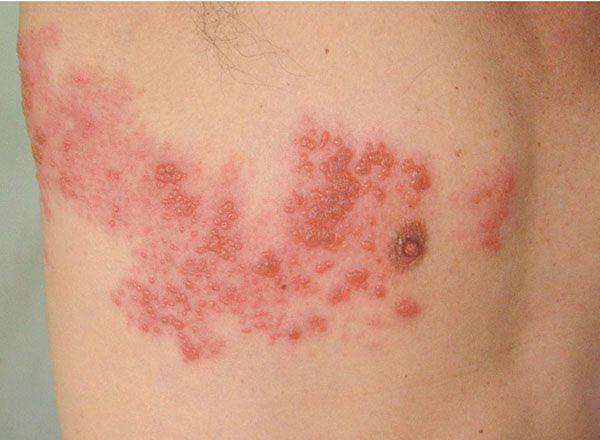
Figure 25-8 Herpes zoster (shingles). Vesicles with a characteristic dermatomal distribution on the right chest and back.
Herpes Zoster in Immunocompromised Hosts
Both the incidence and the severity of herpes zoster are greater in patients with impaired cellular immunity. The incidence of herpes zoster is particularly high in patients with advanced Hodgkin disease who are receiving chemotherapy or radiation, and in persons infected with HIV. In the context of HIV infection, herpes zoster may fail to resolve, may disseminate, or may produce atypical vegetative and ulcerative lesions (41). Although patients with disseminated herpes zoster but without associated serious illness have good prognoses, patients with impaired cellular immunity may develop widespread, fatal systemic manifestations, such as pneumonia, gastroenteritis, or encephalitis.
Histopathology. Lesions of varicella and herpes zoster are histologically indistinguishable from those of herpes simplex (Fig. 25-4). Frequently, however, the degree of vessel damage, microthrombi, and hemorrhage are more pronounced in varicella and, particularly, in herpes zoster than in herpes simplex. Herpes zoster vasculitis may mimic giant cell arteritis (42). In severe cases of varicella and in disseminated herpes zoster, eosinophilic inclusion bodies have also been observed in the dermis within the nuclei of capillary endothelial cells and of fibroblasts bordering the affected vessels. In contrast, in localized herpes zoster, in which the virus reaches the epidermis by way of the cutaneous nerves rather than the capillaries, inclusion bodies have been demonstrated within neurilemmal cells of the small nerves in the dermis underlying the vesicles. Immunohistochemical methods using specific monoclonal antibodies may have some utility in separating VZV from HSV. Using an antibody specific for an envelope glycoprotein, Nikkels and colleagues demonstrated VZV reactivity in sebaceous cells, endothelial cells, mononuclear phagocytes, and factor XIIIa–positive dendrocytes (43).
Tzanck Smear. Cytologic examination of the contents of vesicles is carried out for varicella and herpes zoster in the same way as for herpes simplex. It is a very useful diagnostic test, confirming the diagnosis in 80% to 100% of the cases, whereas viral cultures can confirm in only 60% to 64% (44).
Histogenesis and Viral Identification. Cultures remain the gold standard for virologic confirmation, and the virus can be cultured easily from vesicular fluid within 5 days of the onset of symptoms or before the crusting of lesions. However, culture is not very sensitive, and in contrast with herpes simplex, the VZV does not grow in ordinary tissue cultures; it grows only in human fetal diploid kidney cells or human foreskin fibroblasts. Specific antibodies are available for serologic or immunohistochemical viral identification, and rapid qualitative PCR-based detection of VZV DNA in clinical specimens is also available in many laboratories. Electron microscopic examination of the cutaneous lesions of varicella and herpes zoster reveals virus particles in the capillary endothelium in varicella and sporadically in the axons of dermal nerves in herpes zoster. The HSV and the VZV are indistinguishable by electron microscopy.
Principles of Management. Varicella infection can be prevented or diminished by age appropriate vaccine, either in childhood or with the zoster vaccine at age 60. Once varicella infection occurs, the mainstay of treatment is antiviral medications and supportive pain relief. Generally acyclovir or valacyclovir is used as a first-line agent. Systemic oral or intravenous antiviral treatments are required for the treatment of adult chickenpox and herpes zoster (45). Recently, herpes zoster vaccine in older population has been shown to reduce the incidence of herpes zoster and postherpetic neuralgia (46). Immunosuppressed patients or those with widespread infection may benefit from parenteral administration, and should be evaluated for liver or lung involvement. Patients with active varicella infection are contagious and should be advised to avoid pregnant and immunosuppressed people. Hospitalized patients with active zoster should be placed on contact isolation; patients with disseminated disease require airborne and contact isolation, as the varicella virus can disseminate to the lungs and may be aerosolized.
Cytomegalovirus
Clinical Summary. CMV is a herpes virus that is ubiquitous in human populations. It has been estimated that 1% of newborns are infected transplacentally, and about 5% more acquire the infection at the time of birth. There is a steady increase with age in the number of individuals with antibodies in the serum, and this number takes a significant jump at the time of adolescence, presumably due to transfer of virus through the oral route (47). CMV may also be sexually transmitted. Owing to its ability to remain latent in peripheral leukocytes, CMV can be transmitted via transfusion. Most individuals have a mild flulike episode upon initial infection; however, the virus subsequently becomes latent and persists in many tissues in the body. In immunocompromised individuals, reactivation and involvement of almost any organ of the body can take place and produce a widespread, potentially fatal, systemic infection (48).
Skin manifestation of CMV infection is rare in immunocompetent persons. Cutaneous lesions reported in the immunocompromised patients vary greatly, from genital ulceration to ulceration of the chest, and a more or less widespread maculopapular rash that may become purpuric, sharply punched-out ulcers, urticaria, vesiculobullous lesions, keratotic lesions, and epidermolysis have been reported (49). In the context of HIV infection, CMV-associated viral cytopathic changes can be observed as epiphenomena in skin biopsies in that they do not appear to be the primary pathogen in the specimen and the cause of the skin eruption.
Neonates with congenital CMV infection may exhibit the clinical picture of the “blueberry muffin baby” at delivery which is manifested by petechiae and purpuric magenta-colored macules, papules, and plaques, as well as blueberry-colored ecchymoses. The hemorrhagic (purpuric) skin lesions are thought to reflect extramedullary hematopoiesis. Ultrastructural study demonstrates that complexes of red cells in various stages of maturation occur in the skin of these patients, similar to the erythroblastic islands in the bone marrow (50). Transplacental transfer of CMV is one of the causes of TORCH, a congenital infection caused by Toxoplasma, Rubella, CMV, or Herpesvirus, with extensive damage to the developing brain, chorioretinitis, and pneumonitis (51).
Histopathology. Dilated dermal vessels exhibit, among normal endothelial cells, large, irregularly shaped endothelial cells with large, hyperchromatic, basophilic, intranuclear inclusions that are around 10 μm in size, as well as small intracytoplasmic inclusions (around 3 μm in size, see Fig. 25-9). Some of the inclusions are surrounded by clear halos. A mixed inflammatory infiltrate with focal leukocytoclastic changes may also be present. In the context of HIV infection, other pathologic processes may also be present in the same tissue section.
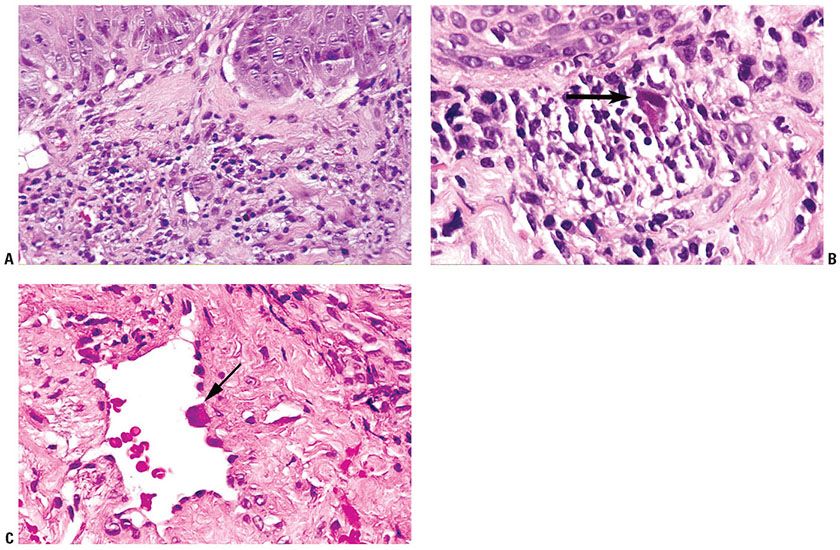
Figure 25-9 Cytomegalic inclusion disease. A: Scanning magnification shows superficial perivascular and diffuse inflammation with prominent vessels. Enlarged endothelial cells may be apparent at this magnification. B: High magnification, one infected dermal cell in the center shows enlarged, hyperchromatic, basophilic, intranuclear inclusions. There is a mixed inflammatory infiltrate in the dermis. C: Dilated dermal vessel with large, irregularly-shaped endothelial cells. The enlarged endothelial cells contain hyperchromatic, basophilic, intranuclear inclusions (arrow points to an inclusion). There may be basophilic cytoplasmic inclusions also.
Histogenesis and Viral Identification. CMV culture can be performed using human fibroblasts. PP65 is a CMV viral protein that is detectable in infected peripheral mononuclear cells, which is a sensitive method to estimate the systemic viral load (52). Immunohistochemical studies using monoclonal or polyclonal antibodies to CMV antigens can be used in paraffin-embedded sections to reveal viral proteins and confirm the presence of the virus. CMV DNA can also be amplified from skin biopsy specimens with PCR using specific primers for CMV, or can be identified by in situ hybridization. Electron microscopy shows intranuclear viral particles approximately 110 nm in diameter. The virus greatly resembles that of herpes simplex.
Principles of Management. CMV infection in immunocompromised patients may be devastating and is generally managed in conjunction with an infectious disease specialist; treatment usually revolves around ganciclovir. Ganciclovir, a deoxyguanosine analog, which is also a DNA polymerase inhibitor, is used for CMV infection as a monotherapy or in combination with immunoglobulin. Foscanet is approved for therapy for CMV retinitis in AIDS patients (53).
Epstein–Barr Virus
Clinical Summary. The Epstein–Barr virus (EBV) is thought to be responsible for a number of diseases such as IM, oral hairy leukoplakia, and cutaneous lymphoproliferative disorders, as well as Burkett lymphoma and nasopharyngeal carcinoma (54). Infection with the EBV develops first in the salivary gland. Large amounts of the virus are released in the saliva, enabling it to spread from one person to another. People infected with the EBV will retain it for life and most are asymptomatic. It has been estimated that the virus infects almost everyone in developing countries and more than 80% of people in developed countries. Most people are infected with the virus during childhood, and are usually not noticeably affected. On the other hand, people infected for the first time during or after adolescence (10% to 20% of people living in developed countries) have a 50% chance of developing IM. The main site of viral persistence is within latently infected lymphocytes, although infectious virus is also released into saliva from infected cells in the oropharynx (55).
IM is a self-limited manifestation of acute EBV infection. The transient rash that occurs quite commonly in patients with IM who have received antibiotic therapy is an erythematous, maculopapular eruption, usually located on the trunk and upper extremities. The palms, soles, and oral mucosa also have been reported to be occasionally involved. The pathogenesis of the rash is believed to be secondary to polyclonal B-cell activation that occurs during EBV infection with production of polyclonal antibodies that form immune complexes capable of fixing complement (56).
Oral hairy leukoplakia is another manifestation of EBV infection that occurs in immunosuppressed HIV-positive and HIV-negative individuals. In HIV-positive individuals, it serves as an indicator of disease severity and rapid progression to AIDS. The presence of oral hairy leukoplakia in an individual should prompt the clinician to perform a thorough history-taking and investigation of immune status. These lesions characteristically resemble whitish patches with a verrucous or filiform irregular surface that cannot be scraped off and are located primarily on the lateral surfaces of the tongue unilaterally or bilaterally. The condition is usually asymptomatic, but in some cases a burning sensation can occur, which is most likely secondary to coinfection by Candida (57). Lymphoproliferative disorders associated with EBV are well documented in the literature.
Chronic EBV infection has been associated with hydroa vacciniforme–like cutaneous T-cell lymphoma occurring mainly in children (58). In addition, posttransplant lymphoproliferative disease, extranodal NK/T-cell lymphoma and cutaneous angiocentric lymphoma have been shown to be associated with EBV infection (59,60).
Histopathology. The histology of the macular lesion associated with IM is nonspecific and usually shows superficial perivascular lymphohistiocytic infiltrates. The histologic features that typify oral hairy leukoplakia include irregular epithelial hyperplasia with associated parakeratosis and acanthosis (Fig. 25-10A). Ballooning of keratinocytes occurs, and there are small, pyknotic nuclei with intranuclear inclusions and a clear perinuclear halo. The cytoplasm may adopt a ground-glass appearance (Fig. 25-10B). There is also a mild inflammatory infiltrate observed in the superficial dermis. Langerhans cells are noted to be absent in the epithelium. Hyphae of Candida are observed within the superficial epithelium of a majority of the patients with oral hairy leukoplakia and may play a role in the development of the epithelial hyperplasia observed in oral hairy leukoplakia. A leukocytoclastic vasculitis and neuropathy associated with chronic EBV infection have also been reported. EBV can be detected by in situ hybridization (Fig. 25-10C).
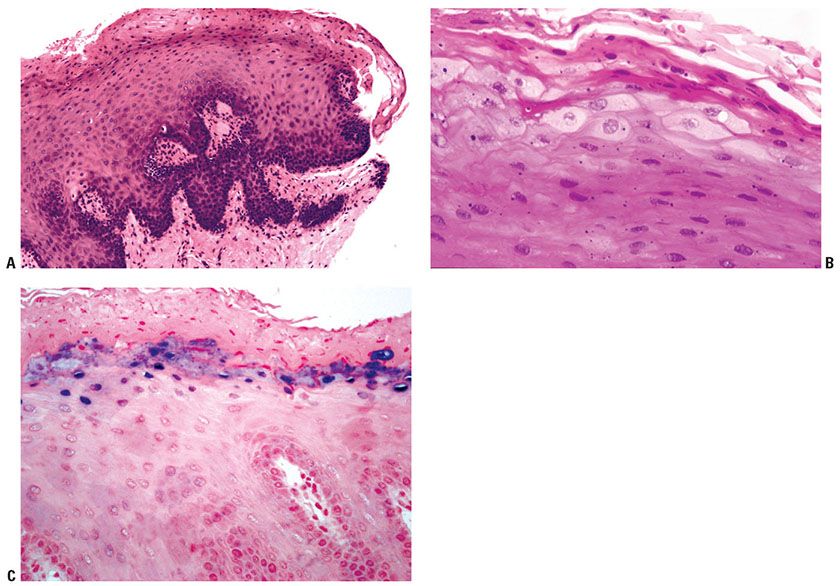
Figure 25-10 Oral hairy leukoplakia. A: Scanning magnification shows the characteristic complex rete pattern of lingual epithelium. There is hyperkeratosis and parakeratosis, and there may be neutrophils in the stratum corneum. Pallor of superficial keratinocytes is focally apparent. B: Detail of swollen, pale superficial keratinocytes, with open pale nuclei, but without specific viral cytopathic changes and scant inflammation in the superficial lamina propria. C: In situ hybridization shows EBV in the superficial keratinocytes.
Principles of Management. Patients with EBV infection generally require supportive care for acute disease; EBV-associated secondary processes require directed therapy, which will vary by disease type.
Human Herpesvirus-6, -7 and -8
Clinical Summary. Human herpesviruses 6, 7, and 8 may cause primary or chronic persistent infection or remain in a state of latency for many years, until a decrease in the immunologic state of the host leads to reactivation of infection (61). HHV-6 was first isolated in 1986 from patients with lymphoproliferative disorders and was classified into two variants: HHV-6A and HHV-6B. HHV-6B is the causative agent of exanthem subitum. Children start to be infected with HHV-6 at 6 months of age and almost all have antibodies by 2 years of age. The transmission of HHV-6 is essentially by a salivary route (62). HHV-6 infects latently after the primary infection and reactivates especially under an immunosuppressive condition. Skin rash in the first month after allogeneic bone marrow transplant (63), generalized vesiculobullous eruptions after allogeneic bone marrow transplantation (64), Papular-purpuric “gloves-and-socks” syndrome (65), Gianotti–Crosti syndrome (66), and a serious systemic reaction to a limited number of drugs called hypersensitivity syndrome have been associated with HHV-6 (67). Drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome, also referred to as drug-induced hypersensitivity syndrome, has been reported to be associated with the reactivation of herpesviruses, especially HHV-6, although CMV, EBV, and HHV-7 are also implicated (68). Some suggest that in genetically predisposed hosts, exposure to a drug triggers latent virus replication and then the patient suffers from an exuberant host antiviral response leading to the clinical phenotype of DRESS and some of the delayed autoimmune sequela. HHV-6 has also been detected in skin biopsy samples of patients with graft-versus-host disease, pityriasis rosea, and lymphoproliferative malignancies.
HHV-7, isolated from CD4+ T lymphocytes from the peripheral blood of a healthy individual, has been recognized as a new lymphotropic herpesvirus. Healthy adults frequently shed the virus into saliva, and children are infected at a young age but somewhat later than HHV-6B. Latency is established in peripheral blood T cells, and a persistent infection in the salivary glands is believed to be the most likely mode of transmission. The primary infection with HHV-7 is linked to febrile illness with or without rash that resembles exanthema subitum. Pityriasis rosea is thought to be associated with HHV-7; however, the association is still controversial (69).
HHV-8 was first identified in tissue samples of patients with Kaposi sarcoma (KS) associated with AIDS in 1994. Serologic studies have demonstrated that, unlike other human herpesviruses, HHV-8 is not ubiquitous; instead, its infection rates parallel the incidence of KS in the region. HHV-8 spreads through exchange of saliva and other sexual activities. HHV-8 can be found in all types of KS, whether related to HIV or not. HHV-8 seems to resemble EBV in its possible transforming properties and HHV-8 is clearly associated with KS, multicentric Castleman disease, and primary effusion lymphoma (70). There may be a synergistic relationship between the HIV-1 Tat protein and HHV-8 infection. Tat promotes the growth of the KS spindle cells. Like other herpesviruses, HHV-8 establishes latent and persistent infection. Only a minority of infected cells yields infectious viral particles, and their role in the development of KS and other associated diseases has not been clearly established. Presence of virus can be established in mononuclear cells of peripheral blood, endothelial cells and spindle cells within skin lesions.
Histopathology. Exanthem subitum is linked to HHV-6 infection. It is a form of superficial perivascular dermatitis with papillary dermal edema. Exocytosis of lymphocytes is often present. Inclusions similar to those in herpes simplex infection are not seen. Histopathology of KS is described in Chapter 34.
Principles of Management. Skin diseases caused by HHV-6 and HHV-7 only need symptomatic and supportive management, and there are currently no approved treatments for them (71).
POXVIRIDAE
The Poxviridae are double-stranded DNA virus with two subfamilies and 11 genera. Poxvirus infections of humans include variola (smallpox), cowpox, vaccinia, molluscum contagiosum, paravaccinia (Milker’s nodule), and orf (ecthyma contagiosum).
Variola (Smallpox)
Clinical Summary. Smallpox was one of the most infectious and deadly diseases in the world, afflicting millions of people each year regardless of age, race, or socioeconomic status before it was eradicated in 1977 (72). There were two forms of smallpox: variola major and variola minor. Variola major had a case fatality rate of approximately 25%, while variola minor, a less virulent form, had a case fatality rate below 1%. Although the two forms caused disease of different severity, they were indistinguishable from one another. Smallpox was spread through respiratory transmission of virus found in the oropharyngeal secretions of infected individuals. Penetration was usually through the respiratory tract and local lymph nodes, and then the virus entered the blood (primary viremia). Internal organs were infected; then the virus reentered the blood (secondary viremia) and spread to the skin. The onset of smallpox was acute, with fever, malaise, headaches, and backaches. The initial toxemia phase lasted 4 to 5 days. On about the third or fourth day, the characteristic rash appeared. First, it appeared on the buccal and pharyngeal mucosa, the face, and the forearms. Within a day, it spread to the trunk and lower limbs. The lesions of the rash began first as macules, which soon became papules, and then developed into pustular vesicles. The lesions usually protruded from the skin and they were firm to touch. The lesions dried up and often became crusted by day 14. By the end of the third week, most crusts had fallen off, with the exception of the palms and the soles (73). This entire process took 3 to 4 weeks, and the areas affected by the rash could be permanently scarred. The rare hemorrhagic-type smallpox that appeared in immunocompromised individuals was associated with bleeding from the conjunctiva and mucous membranes, very severe toxemia, and early death, usually before the lesions of the skin had developed. Modified-type smallpox was seen in persons who had been vaccinated, usually many years earlier. In these cases, the skin lesions evolved quickly and they were more variable in size.
Histopathology. There is prominent ballooning and reticular degeneration. Under light microscopy, aggregations of variola virus particles, called Guarnieri bodies, may be found in cytoplasm; occasional intranuclear inclusions can also be found. Similar changes can be seen in vaccinia, monkeypox, or cowpox.
Vaccinia
Clinical Summary. Vaccinia virus was used for smallpox vaccination via inoculation into the superficial layers of the skin of the upper arm. However, with the eradication of smallpox, routine vaccination with vaccinia virus ceased for many years, but may soon become more common because of security concerns. Four to five days following vaccination with vaccinia virus, a papule appears at the site of vaccination. Two or three days later the papular lesion becomes vesicular, growing until it reaches its maximum diameter on the 9th or 10th day. The lesion dries from the center outward, and the brown scab falls off after about 3 weeks, leaving a scar—a mark by which previous vaccines can be recognized (74). Complications of vaccination can occur but are very rare, including the following conditions: progressive vaccinia (vaccinia necrosum), which is a severe, potentially fatal illness characterized by progressive necrosis at the site of vaccination; eczema vaccinatum, which is similar to eczema herpeticum in patients with chronic eczema; generalized vaccinia, which is characterized by a vesicular rash that sometimes covered the entire body; and postvaccinial encephalitis, a neurologic complication in the most serious ones that occurred from vaccination with vaccinia virus (75). Other localized lesions such as keloid, dermatofibrosarcoma protuberans, and malignant fibrous histiocytoma have been reported at the site of inoculation. These complications are extremely rare.
Histopathology. The histologic changes are similar to those of herpes simplex and zoster and varicella. However, intracytoplasmic inclusions are seen in vaccinia, unlike in herpes infection.
Human Cowpox Infection
Clinical Summary. Despite its name, the reservoir hosts of cowpox virus are rodents, from which it can occasionally spread to cats, cows, humans, and zoo animals, including large cats and elephants. Infection in these animals is usually manifested as a single, small, crusted ulcer. Transmission to humans has traditionally occurred via contact with the infected teats of milking cows. However, currently, infection is seen more commonly among domestic cats, from which it can be transmitted to humans. Patients present with painful, hemorrhagic pustules or black eschars, usually on the hand or face, accompanied by edema, erythema, lymphadenopathy, and systemic involvement (76). Crusted lesions resembling anthrax, and sporotrichoid infection have been reported.
Histopathology. The pathology of the skin lesions caused by cowpox virus is similar to that of smallpox, with prominent ballooning and reticular degeneration. However, there is greater epithelial thickening and less rapid cell necrosis. Early lesions of human cowpox infection show prominent reticular degeneration. Eosinophilic, intracytoplasmic inclusion bodies are present, a valuable feature in distinguishing poxvirus from herpesvirus infections. Under electron microscopic examination, the virus is rectangular and morphologically indistinguishable from the virus of variola.
Parapox Virus Infections (Milker’s Nodules and Orf)
Clinical Summary. Milker’s nodules, orf, and bovine papular stomatitis pox are clinically identical in humans and are induced by indistinguishable paravaccinia viruses. Milker’s nodules are acquired from udders infected with pseudocowpox or paravaccinia (parapox). This disease is called bovine papular stomatitis pox when the source of the infection is calves with oral sores contracted through sucking infected udders. Orf (ecthyma contagiosum) is acquired from infected sheep or goats with crusted lesions on the lips and in the mouth.
The most common presentation of Orf is a circumscribed solitary nodule or papule on the fingers or hands (77). Milker’s nodules usually presents with multiple lesions. After an incubation period of 3 to 7 days, parapox virus infections produce one to three (rarely more) painful lesions measuring 1 to 2 cm in diameter on the fingers, or occasionally elsewhere as a result of autoinoculation. During a period of approximately 6 weeks, they pass through six clinical stages, each lasting about 1 week: (a) the maculopapular stage; (b) the target stage, during which the lesions have red centers, white rings, and red halos; (c) the acute weeping stage; (d) the nodular stage, which shows hard, nontender nodules; (e) the papillomatous stage, in which the nodules have irregular surfaces; and (f) the regressive stage, during which the lesions involute without scarring.
Histopathology. During the maculopapular and target stages, there is vacuolization of cells in the upper third of the stratum malpighii, leading to multilocular vesicles. Eosinophilic inclusion bodies are in the cytoplasm of vacuolated epidermal cells, a distinguishing feature from herpes virus infections (Fig. 25-11C). Intranuclear eosinophilic inclusion bodies are also present in some cases. During the target stage, vacuolated epidermal cells with inclusion bodies are only in the surrounding white ring. Ballooning degeneration occurs, the affected keratinocytes rupture and the resulting defects have a tendency to coalesce and to produce reticulated vesicles (Fig. 25-11A, B). The epidermis shows elongation of the rete ridges, and the dermis contains many newly formed, dilated capillaries and a mononuclear infiltrate.
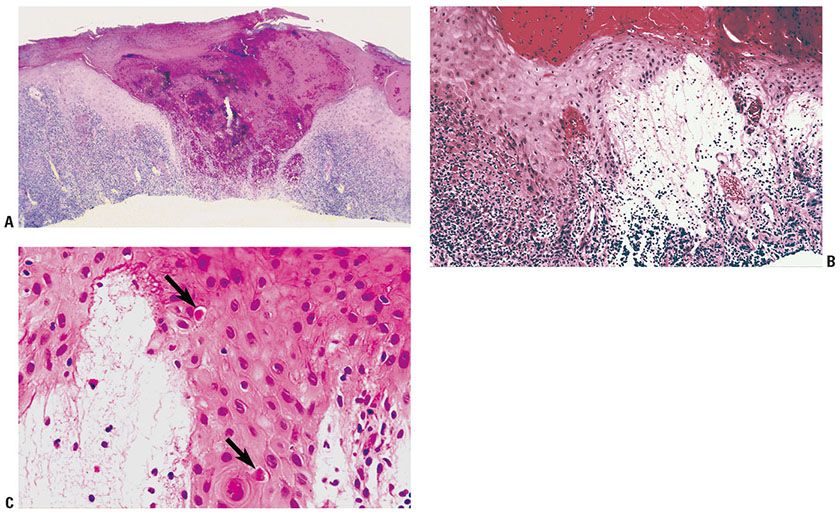
Figure 25-11 Orf. A:
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








