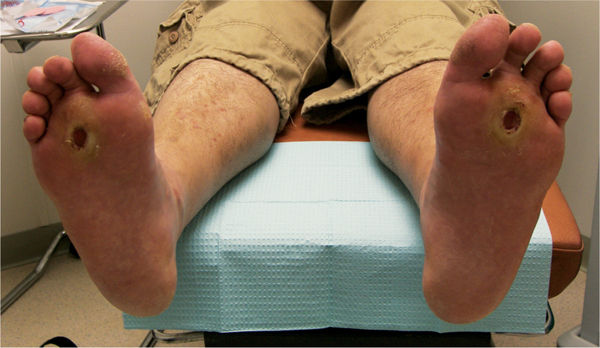
FIGURE 7-1 Statistics on the prevalence of diabetes in the United States show evidence that it is an epidemic disease that accounts for over 30% of the Medicare budget for health care.5
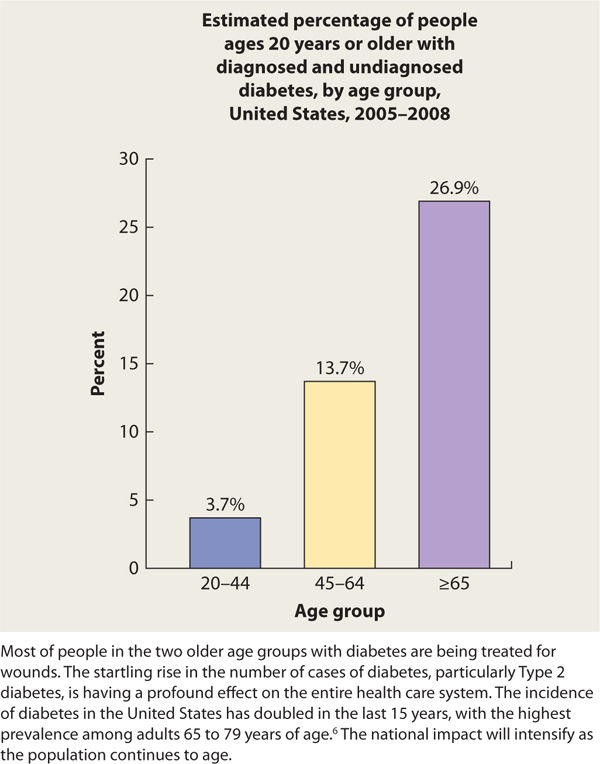
Diabetes has a profound impact on health as a result of the secondary complications such as heart disease, kidney disease, retinopathy, neuropathy, and lower-limb amputation. In addition, people are acquiring T2DM at younger ages than ever before in the United States.5 Therefore, T2DM is no longer a disease for only the over 40 age group. Accordingly, providers are seeing younger people with diabetes-related complications, including issues related to foot pathology and wound healing challenges. Furthermore, the older population is being affected the most from a numbers perspective, with the largest prevalence of diabetes in the >65-year-old age group (TABLE 7-1).5
TABLE 7-1 Prevalence of Diabetes in Three Age Groups
Health care professionals who care for people with chronic wounds who also have diabetes are challenged to achieve wound closure and optimal wound healing outcomes. Diabetes management strategies and wound/foot care are both necessary to provide an environment in which wound closure can occur.
The total annual cost of diabetes treatment in 2002 (including direct and indirect costs) was estimated at $132 billion, or one out of every 10 health care dollars spent in the United States.6
Effects of Diabetes on Wound Healing
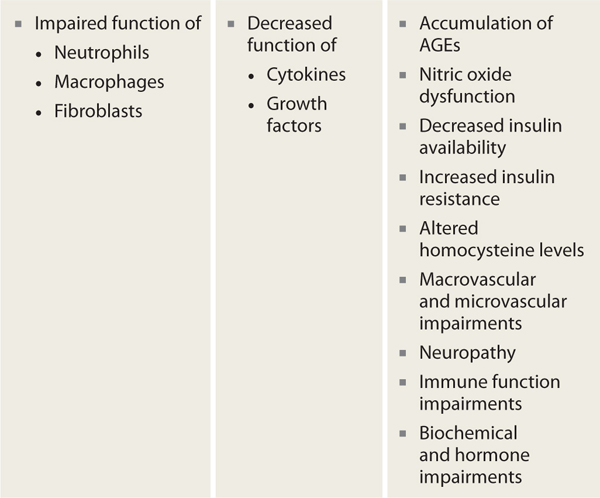
Faulty wound healing is a well-recognized complication of diabetes. Current evidence confirms that diabetes inhibits all phases of wound healing via impaired function of the primary cells responsible for wound repair (ie, neutrophils, macrophages, and fibroblasts), frequently resulting in delayed healing or chronic nonhealing wounds. In addition, there is decreased efficacy of cytokines and growth factors in people with diabetes and accompanying hyperglycemia. The accumulation of advanced glycosolated end products (AGEs), nitric oxide dysfunction, decreased insulin availability or increased insulin resistance, and altered homocysteine levels also contribute to the complex healing impairments for people with diabetes. Micro- and macrovascular disease, neuropathy, immune dysfunction, and biochemical and hormonal abnormalities all contribute to the altered tissue repair processes in people with diabetes and hyperglycemia (TABLE 7-6).8
TABLE 7-6 Mechanisms by Which Diabetes Impairs Wound Healing
One example of a diabetes-mediated impairment in wound healing is susceptibility to infection. Under normal conditions, during the hemostasis healing phase there is immediate fibrin plug formation as platelets aggregate at the wound site. The platelets release various growth factors and cytokines, which then recruit inflammatory cells; however, in a hyperglycemic environment, there is a delay in fibrin plug formation, leaving the wound open to contaminants. In addition, there is a delay (or decrease) in the release of growth factors and cytokines, causing impaired recruitment of the inflammatory cells. With this delay, the individual is at risk for infection. In fact, people with diabetes have more frequent infections than patients without diabetes.8
Research in human and animal models has identified many of the changes that contribute to delayed wound healing at the molecular level; however, more research is needed to completely understand how diabetes contributes to faulty tissue repair.9
Extensive research has focused on the causes of and interventions for diabetic (also referred to as neuropathic) foot wounds; however, the impairments related to diabetes are much more far-reaching than just the foot, affecting healing of all types of wounds regardless of etiology (for example, pressure ulcers, vascular ulcers, and surgical wounds). In summary, diabetes profoundly impairs the four overlapping phases of wound healing. The underlying mechanisms of the effects of diabetes on wound healing have been extensively investigated over the past few decades. However, complete understanding of the complex and multifaceted pathophysiologic relationship between DM and defective healing continues to elude the scientific community.
Medical Management and Team Care of Diabetes
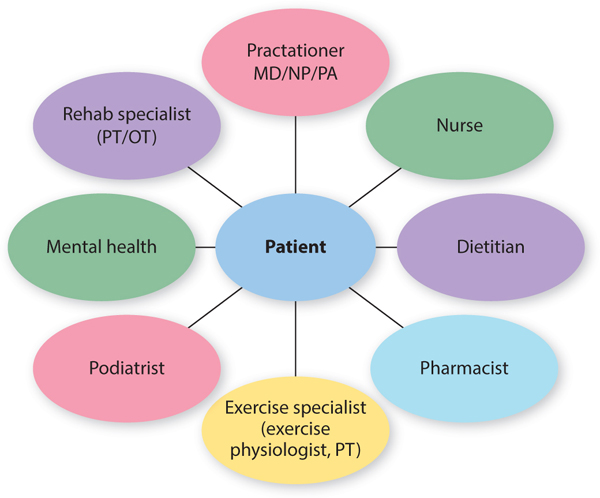
Diabetes care is complex and multifaceted, requiring a team approach to patient-centered care, with the patient being an integral member of the team (FIGURE 7-9). While the medical team leader is the physician or advanced practice nurse (who uses input, education services, and treatments from other health care providers), the management that occurs on a daily basis is provided by the patient (or by caregivers when the patient is too impaired to perform adequate self-management).9 The ability of the patient to adequately manage the diabetes depends on extensive and ongoing training, education, and medical feedback.
FIGURE 7-9 The diabetes care team The team that cares for the patient with diabetes includes these health care specialists, each with an important role in caring for the total patient, with the patient being at the center of the team. The team must have the patient take responsibility for the overall day-to-day management of the disease.
Effective wound care includes a review of the patient’s diabetes management strategies. Without good glucose control, the patient will have difficulty reaching wound closure and obtaining the desired healing and functional goals.
The following list provides the basic elements of a well-rounded diabetes management program:
 Diabetes self-management education/training (DSME/T): Diabetes self-management education/training is defined by the American Association of Diabetes Educators (AADE) as a collaborative process through which people with or at risk for diabetes gain the knowledge and skills needed to modify behavior and successfully self-manage the disease and its related conditions. This intervention aims to achieve optimal health status, to achieve better quality of life, and to reduce the need for costly health care.10 The process incorporates the needs, goals, and life experiences of the person with DM and is guided by evidence-based standards of care. Informed decision making and problem solving become core components of the implementation process for DSME/T. DSME/T strategies should be used in active collaboration with the health care team, with the outcome being the maintenance or improvement of quality of life and avoidance of diabetes complications.1,5 TABLE 7-7 lists the content areas for DSME/T, all of which should be incorporated into a well-designed education program.
Diabetes self-management education/training (DSME/T): Diabetes self-management education/training is defined by the American Association of Diabetes Educators (AADE) as a collaborative process through which people with or at risk for diabetes gain the knowledge and skills needed to modify behavior and successfully self-manage the disease and its related conditions. This intervention aims to achieve optimal health status, to achieve better quality of life, and to reduce the need for costly health care.10 The process incorporates the needs, goals, and life experiences of the person with DM and is guided by evidence-based standards of care. Informed decision making and problem solving become core components of the implementation process for DSME/T. DSME/T strategies should be used in active collaboration with the health care team, with the outcome being the maintenance or improvement of quality of life and avoidance of diabetes complications.1,5 TABLE 7-7 lists the content areas for DSME/T, all of which should be incorporated into a well-designed education program.
TABLE 7-7 Diabetes Education Content Areas10
 Medical nutrition therapy (MNT): MNT is not just a “diet” but rather a comprehensive approach to eating to which the person with diabetes adheres in order to achieve optimal blood glucose control with weight control as a secondary outcome. The term diabetic diet is no longer used.
Medical nutrition therapy (MNT): MNT is not just a “diet” but rather a comprehensive approach to eating to which the person with diabetes adheres in order to achieve optimal blood glucose control with weight control as a secondary outcome. The term diabetic diet is no longer used.
 Physical activity (PA): Physical activity (including exercise) is a powerful modality for the person with diabetes and must be coordinated with the individual’s medication and nutrition regimes. Depending on the associated complications (eg, diabetic retinopathy, diabetic neuropathy, and diabetic nephropathy), certain precautions and contraindications may be implemented to avoid PA-induced damage to already-compromised tissues and organs. For example, patients who have severe foot deformities or a history of DFUs need to be instructed in an exercise program that does not cause friction and shear to the plantar foot structures. A referral to a physical therapist knowledgeable about exercises and diabetes is recommended in order to establish an appropriate exercise program for the patient with diabetes.
Physical activity (PA): Physical activity (including exercise) is a powerful modality for the person with diabetes and must be coordinated with the individual’s medication and nutrition regimes. Depending on the associated complications (eg, diabetic retinopathy, diabetic neuropathy, and diabetic nephropathy), certain precautions and contraindications may be implemented to avoid PA-induced damage to already-compromised tissues and organs. For example, patients who have severe foot deformities or a history of DFUs need to be instructed in an exercise program that does not cause friction and shear to the plantar foot structures. A referral to a physical therapist knowledgeable about exercises and diabetes is recommended in order to establish an appropriate exercise program for the patient with diabetes.
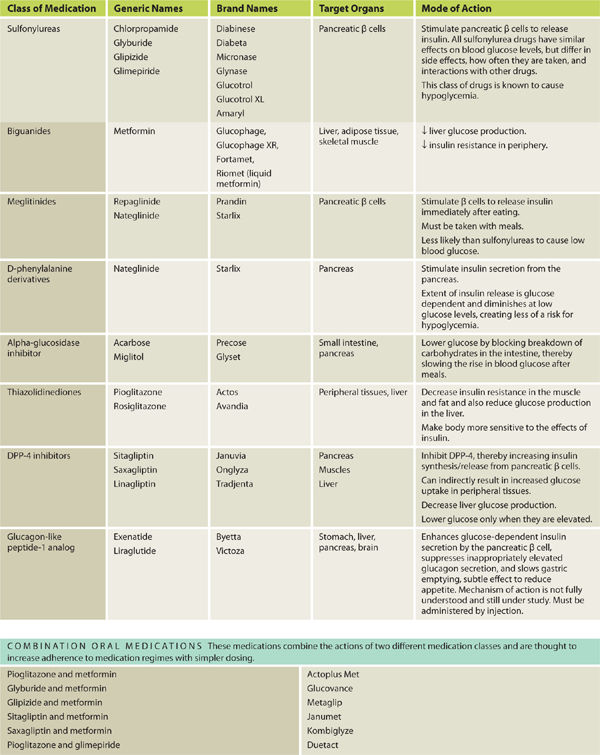
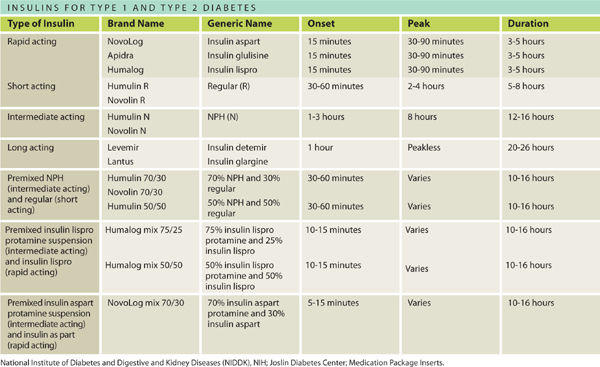
 Pharmacologic management: The drug armamentarium for glycemic control for people with diabetes is large and growing as the impairments related to diabetes are better understood. The different drugs and combination therapies address different pathophysiological mechanisms and different organs involved in blood glucose control. The management of Type 1 diabetes and Type 2 diabetes is completely different, as these are actually different diseases with a similar outcome of hyperglycemia.9 Medications can be oral and/or injectable for Type 2 diabetes; however, Type 1 diabetes requires injectable insulin. Inhaled insulin, a new delivery method for insulin, was approved by the FDA in June 2014 for use by both T1DM and T2DM. Inhaled insulin may take the place of short-acting insulins, but not the intermediate and long-acting forms insulin. See TABLE 7-5.
Pharmacologic management: The drug armamentarium for glycemic control for people with diabetes is large and growing as the impairments related to diabetes are better understood. The different drugs and combination therapies address different pathophysiological mechanisms and different organs involved in blood glucose control. The management of Type 1 diabetes and Type 2 diabetes is completely different, as these are actually different diseases with a similar outcome of hyperglycemia.9 Medications can be oral and/or injectable for Type 2 diabetes; however, Type 1 diabetes requires injectable insulin. Inhaled insulin, a new delivery method for insulin, was approved by the FDA in June 2014 for use by both T1DM and T2DM. Inhaled insulin may take the place of short-acting insulins, but not the intermediate and long-acting forms insulin. See TABLE 7-5.
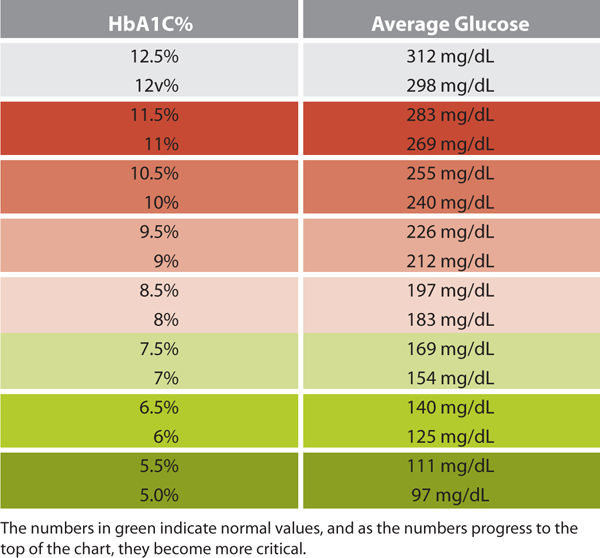
 Glucose monitoring: The most commonly used methods for blood glucose monitoring include capillary blood glucose and the HbA1C test.
Glucose monitoring: The most commonly used methods for blood glucose monitoring include capillary blood glucose and the HbA1C test.
Challenges in Caring for the Patient with Diabetes
Education There are many challenges in providing care for individuals with diabetes. Education is required not only for self-management of diabetes, but also for self-management of the wound. Many people with chronic wounds and diabetes do not have the necessary information to adequately manage the disease, especially in the presence of a chronic wound that causes both psychological and physiological stress. The standard of care for individuals with diabetes is referral to a comprehensive DSME/T program upon diagnosis of the disease9; however, many do not have access to a diabetes education center. Such individuals thus present to the wound care clinic with a critical deficiency in their ability to manage the disease. This deficiency itself could be a contributing factor to both the development of the wound and the impaired wound healing.
Depression and Burnout Many people with diabetes have varying levels of depression,9 which can mildly or severely compromise self-management, depending on their coping abilities. If the individual’s diabetes management strategies are compromised by depression, the provider can expect more challenges for wound healing. Diabetes alone conveys psychological, social, and financial burdens on the affected individual; a wound creates additional psychological, social, and financial burdens.
Burnout is a potential problem for everyone concerned: the patient can become burned out from living with diabetes and its associated complications continuously, with no respite, and the wound care team can become burned out by the impact of diabetes on wound healing and the perception that the patient is not optimally self-managing the disease or the wound, thereby “sabotaging” the wound care plan. The psychosocial impact of diabetes is life altering, especially with the addition of chronic complications that frequently accompany diabetes, including unsightly, odiferous, and difficult-to-manage wounds. There are no easy answers or rote formulas for these challenges; providers must do the best to provide comprehensive support to these patients and their families.9
Adherence The patient’s adherence to the overall disease management plan is critical. However, before placing the term noncompliant or nonadherent on the person with diabetes, the wound care clinician should assess whether the patient has the best diabetes management plan individualized to his or her needs. The clinician must assess the patient’s ability to self-manage the diabetes and provide an appropriate referral as needed to address deficiencies where they exist. The status of the patient’s diabetes control is prerequisite knowledge for the wound care clinician’s ability to help the patient create an environment for wound closure and healing. TABLE 7-8 lists helpful strategies for the clinician who is working with a patient who is having difficulty with adherence.
TABLE 7-8 Strategies to Help a Patient Develop Adherence to a Comprehensive Diabetes Management Program
The Wound Care Clinician’s Role
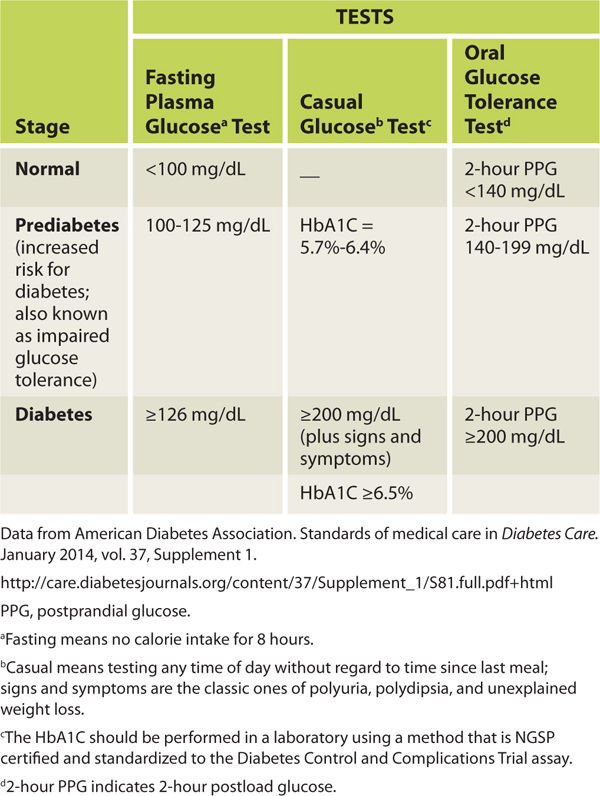
The initial examination of the patient with a wound includes a basic assessment of the patient’s diabetes management plan to ensure that the disease is under control. TABLE 7-9 is a suggested list of intake components to consider, and TABLE 7-10 lists the diagnostic criteria for diabetes.
TABLE 7-9 Important Questions to Ask a Person with Diabetes When Performing the Wound Evaluation
TABLE 7-10 Type 2 Diabetes Diagnostic Criteria for Adults
A typical case scenario may present as follows: a patient with diabetes presents with a wound with signs and symptoms of infection. The blood glucose level is 345 mg/dL. The clinician needs to determine which came first: chronic hyperglycemia, which made the patient more susceptible to infection, or the infection, which caused the blood glucose levels to increase. A comparison of the capillary blood glucose levels with a current HbA1C test can help make this determination. If the HbA1C value is high, then the blood glucose has been out of control for at least several months and diabetes management strategies are of paramount importance. If the HbA1C shows adequate control, then the diabetes has been well managed and the infection has caused the elevated blood glucose levels. At this point, the patient may require the addition of insulin to the diabetes medication regime—at least temporarily—to help control the infection-instigated elevated blood glucose. The wound care clinician can make this recommendation to the provider who manages the patient’s diabetes. Thus object glycemic tests can be used to help manage wound healing, remembering that diabetes is ever present and must be managed at all times to help create the best wound closure and healing outcomes.
Conclusion (Diabetes)
The wound care team needs a working knowledge of the diagnosis of diabetes, diabetes management strategies including DSME/T, most commonly associated complications, and effects of diabetes on wound healing, along with proficiency in performing a thorough assessment of the patient and the wound. While it is not the responsibility of the wound care team to manage the person’s diabetes—that duty belongs to the patient and the practitioner responsible for the medical management—the wound care team assess the disease is being managed as successfully as possible and recognizes when other diabetes interventions and counseling need to be considered for optimal wound closure and healing outcomes.11,12
THE DIABETIC FOOT
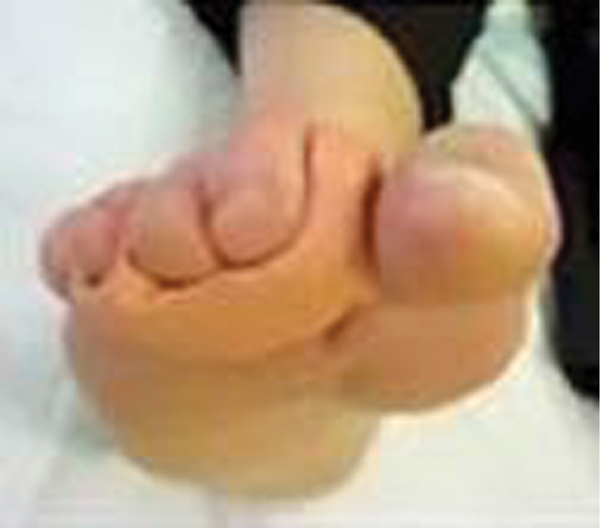
The diabetic foot is a complicated pathological entity suffering from varying degrees of physiological and biomechanical balance and imbalance. Prolonged exposure to slowly elevating blood glucose leads to glycosolation of multiple organ systems and a progressively advancing neurovascular crisis triggered by a loss of system homeostasis. The “perfect storm” of intrinsic and extrinsic stressors associated with diabetes causes a system collapse that results in a breakdown of the foot’s ability to manage the combination of individual stressors, thereby leading to a breakdown sufficient to cause ulceration, or neuroarthropathy of diabetic foot.13
Once the skin has broken and exposed the underlying tissues to the risk of infection, the patient and the practitioner have a relatively short period of time within which to address the component problems and restore balance to the system, otherwise complications such as osteomyelitis or amputation may ensue. Carlyle Begay, in a presentation delivered to the National Indian Health Bureau in January of 2012, referred to this time period as “the golden hour for the diabetic foot,” a concept coined from the emergency management of cardiovascular and cerebrovascular accidents (FIGURE 7-10).14
FIGURE 7-10 Bilateral submetatarsal two ulcers secondary to a short first metatarsal The bilateral plantar wounds on the second metatarsals are a result of a short first metatarsal. These wounds would have a relatively short period of time for treatment before the risk of infection or osteomyelitis is greatly increased.
From the onset of the wound, there are approximately 30 days or 4 weeks to restore homeostasis and thereby prevent further tissue breakdown, infection, and progression to amputation. Standard of care requires the introduction of definitive care before 4 weeks; advanced therapies to heal the wound can be initiated at that time if 50% wound closure has not occurred. Dr. Peter Sheehan conducted a large, prospective, multicenter trial of 203 diabetic patients that assessed the ability of the 4-week healing rate to predict complete healing at 12 weeks. He concluded that “patients in whom ulcer size fails to reduce by half over the first 4 weeks of treatment are unlikely to achieve wound healing over a reasonable period.”15
There is a 5-fold increase in the risk of infection in wounds that progress beyond 4 weeks of therapy and a 155 times greater risk of amputation once an infection develops.16
A panel created for the American Diabetes Association Consensus Development Conference on Diabetic Foot Wound Care in April 1999 concluded that, “Any wound that remains unhealed after 4 weeks is cause for concern, as it is associated with worse outcomes, including amputations.”17 It is evident from these studies that a wound on a diabetic foot is a very serious event and must be treated as such from day one. Early interventions such as risk assessment, educational initiatives, and aggressive treatment interventions can literally be life saving for many patients with diabetes.
Diabetes and the Effects on the Systems of the Foot
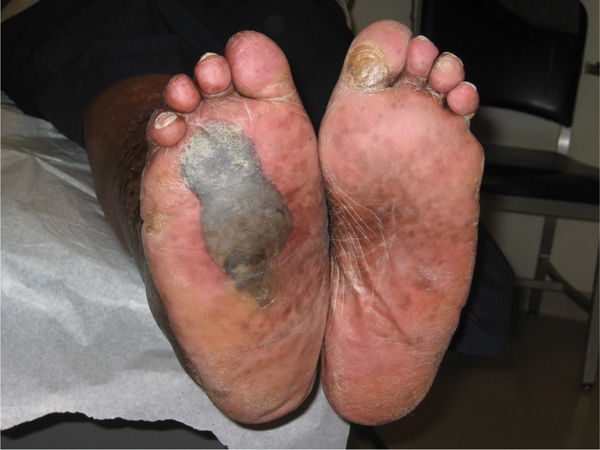
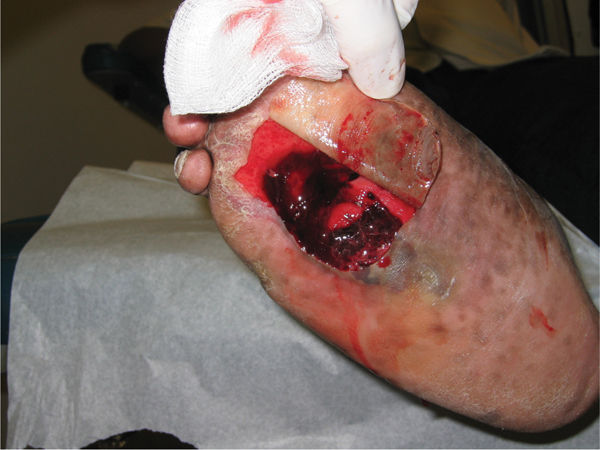
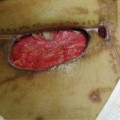
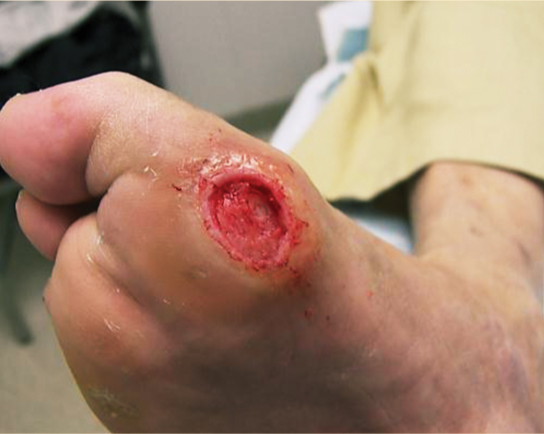
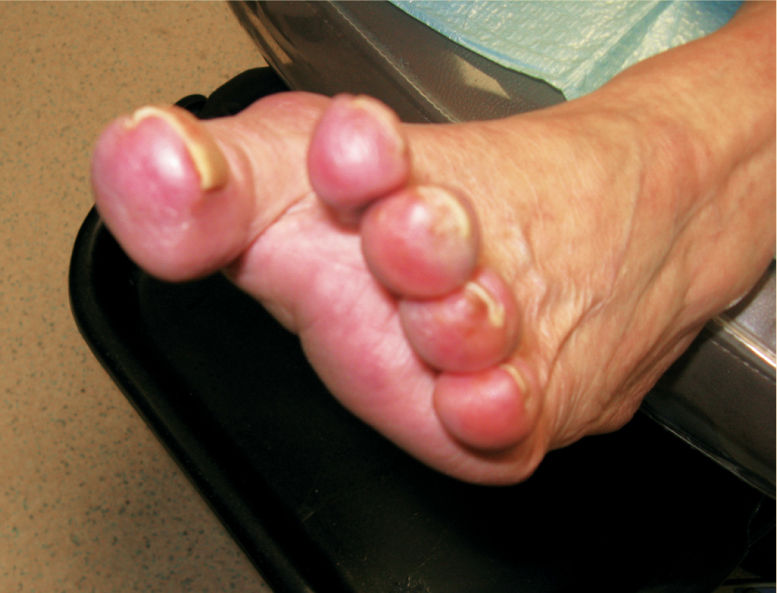
Foot problems are common in patients with diabetes and 25% of them, or approximately 6.8 million people, will develop a foot ulcer during their lifetime.18,19 Once an ulcer has developed, the 5-year survival rate for a patient with diabetes is approximately 45%, a figure that is lower than that for both prostate and breast cancer. Despite the dangers and the risks associated with ulceration in the diabetic population, the problem has received very little press in comparison to other more politically interesting diseases.20 For many years, foot complications have been the leading cause of hospitalization for patients with diabetes.21 Almost 30% of diabetics aged 40 years or older have impaired sensation in the feet (ie, at least one area that lacks feeling), or approximately 8 million individuals (60% to 70% of people with diabetes) have mild-to-severe forms of nervous system damage affecting both sensory and motor systems.22 The major system failure in diabetics leading to the development of an ulcer is the loss of protective sensation in the lower extremity. Combined with intrinsic muscle loss and extrinsic muscle imbalance, this leads to increasing biomechanical stress in areas of high pressure and eventual tissue loss. The skin breakdown is a combination of pressure ischemia, reperfusion injury, expanding sublesional microhemorrhage and micronecrosis, and shearing injury, all of which lead to an accumulation of coalescing blood and transudate within or beneath the skin. The skin eventually ruptures, opening the subcutaneous tissues to bacterial invasion from the skin surface (FIGURES 7-11, 7-12).
FIGURE 7-11 Charcot foot with dissecting sublesional hematoma.
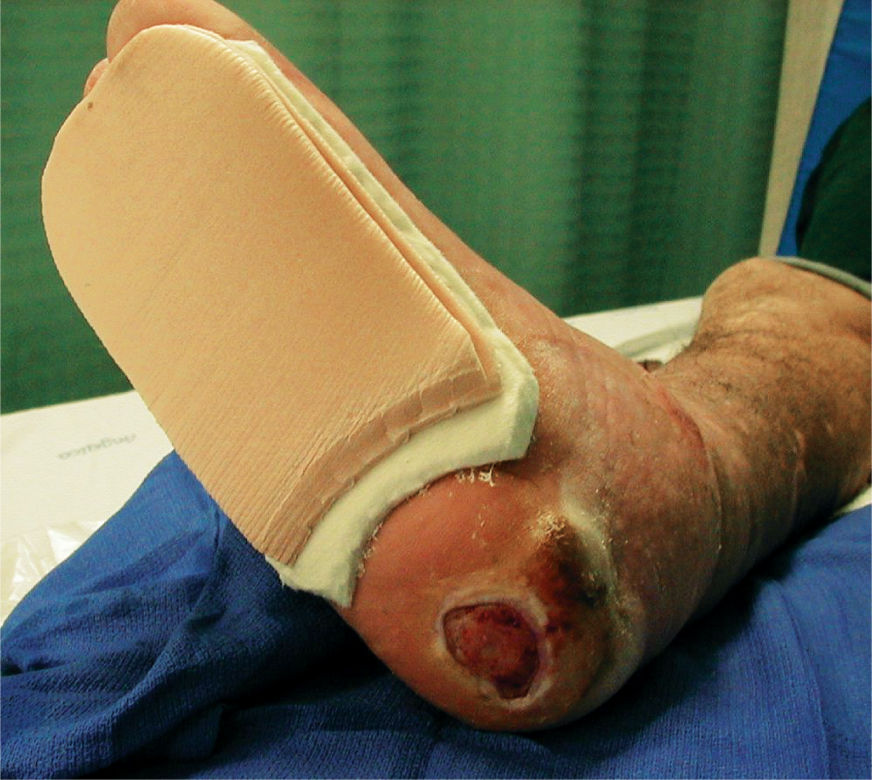
FIGURE 7-12 Deroofed hematoma with exposed full-thickness ulcer.
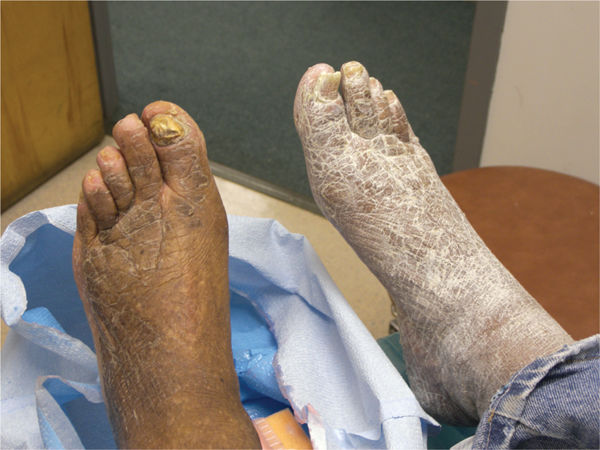
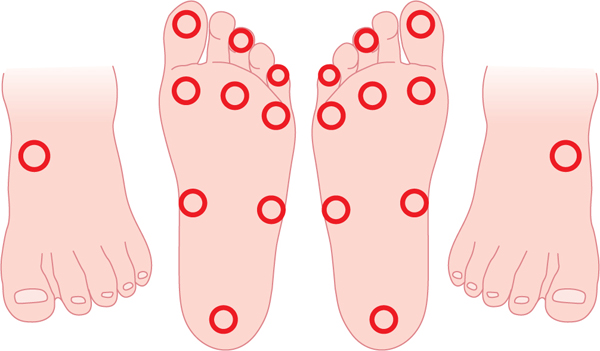
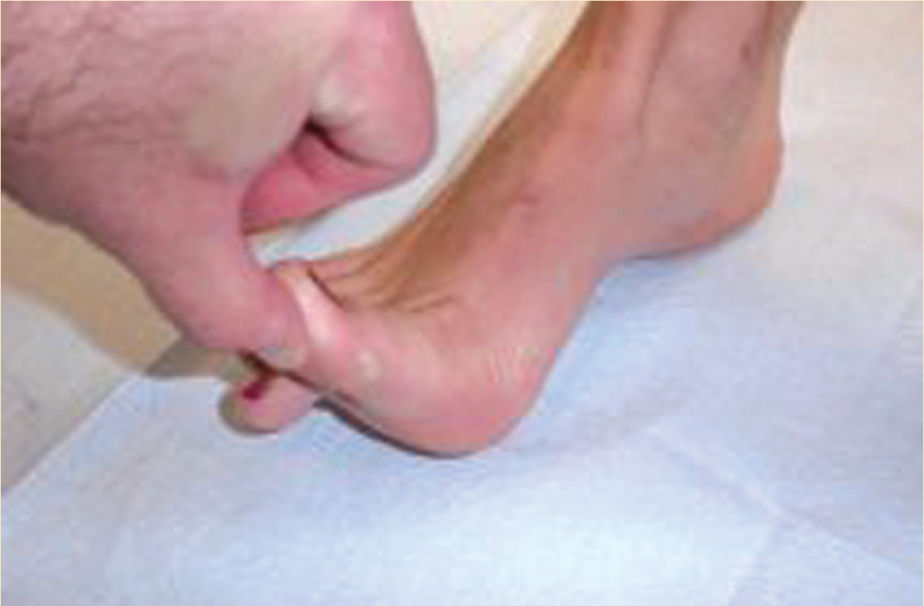
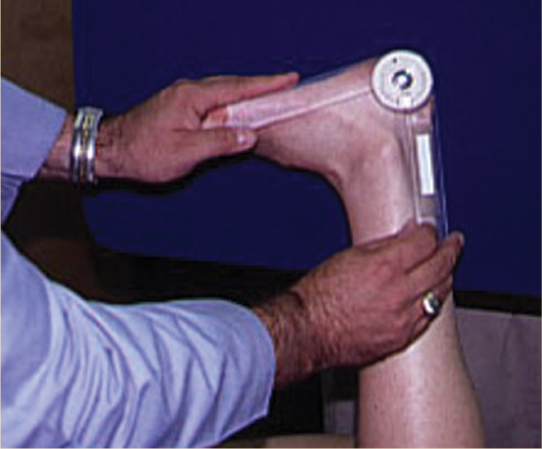
Diabetic dyshidrosis or dry skin secondary to the loss of normal skin perspiring (a result of autonomic nervous dysfunction) is documented, and patient education about the daily use of an emollient is included in the care plan, along with a consultation with a podiatric or allopathic physician for a prescription hydrating cream (FIGURE 7-13). Neurological assessment includes the Semmes-Weinstein monofilament test, reflexes, biothesiometer testing, muscle strength and function, visual gait and posture analysis, and some quantification of plantar pressures (FIGURE 7-8). FIGURE 7-14 illustrates the 10 sites that are tested with the monofilaments. The PressureStat device is an excellent tool for obtaining a quick quantifiable assessment of plantar pressures (FIGURE 7-15).
FIGURE 7-13 Severe dyshidrosis before and after emollient.
FIGURE 7-14 Diagram of sites tested with the Semmes-Weinstein monofilaments Ten sites are tested on each foot to determine the loss of pressure sensation. This is considered one of the most reliable risk factors for diabetic foot ulceration.
FIGURE 7-15 PressureStat pressure mat The pressure mat is used to obtain a quantifiable estimate of dynamic plantar foot pressures.
The major risks for ulceration are determined by the neurological and musculoskeletal findings, and the major risk for amputation is determined by the vascular findings. A complete vascular screening includes pulses, trophic skin changes (eg, hair loss, skin thinning, nail thickening, and pigment changes), capillary refill time, and the calculation of both the ankle brachial index (ABI) and the toe brachial index (TBI). ABIs are often falsely elevated due to calcification of the arteries, leading to artificially elevated vessel occlusion pressures. The toe arteries are much less likely to become calcified, thereby providing a more accurate impression of the true tissue perfusion. (Refer to Chapter 4, Vascular Wounds for more information on vascular testing.)
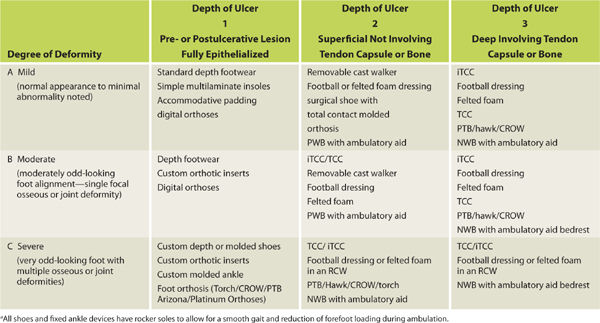
The musculoskeletal assessment of the foot is performed in both weight-bearing and non-weight-bearing positions. Deformities and their severity are assessed along with any motion restrictions, especially restriction produced by the presence of equinus. A simple classification system was developed at Temple University to assess the depth of wounds and the degree of foot deformity in order to determine the type of off-loading device necessary to heal the wound (TABLE 7-11).
TABLE 7-11 Temple University Classification for Footwear Selectiona
Pictorial Glossary of Transitional Off-Loading Devices
See FIGURES 7-16 to 7-28.
FIGURE 7-16 Total contact cast (TCC).
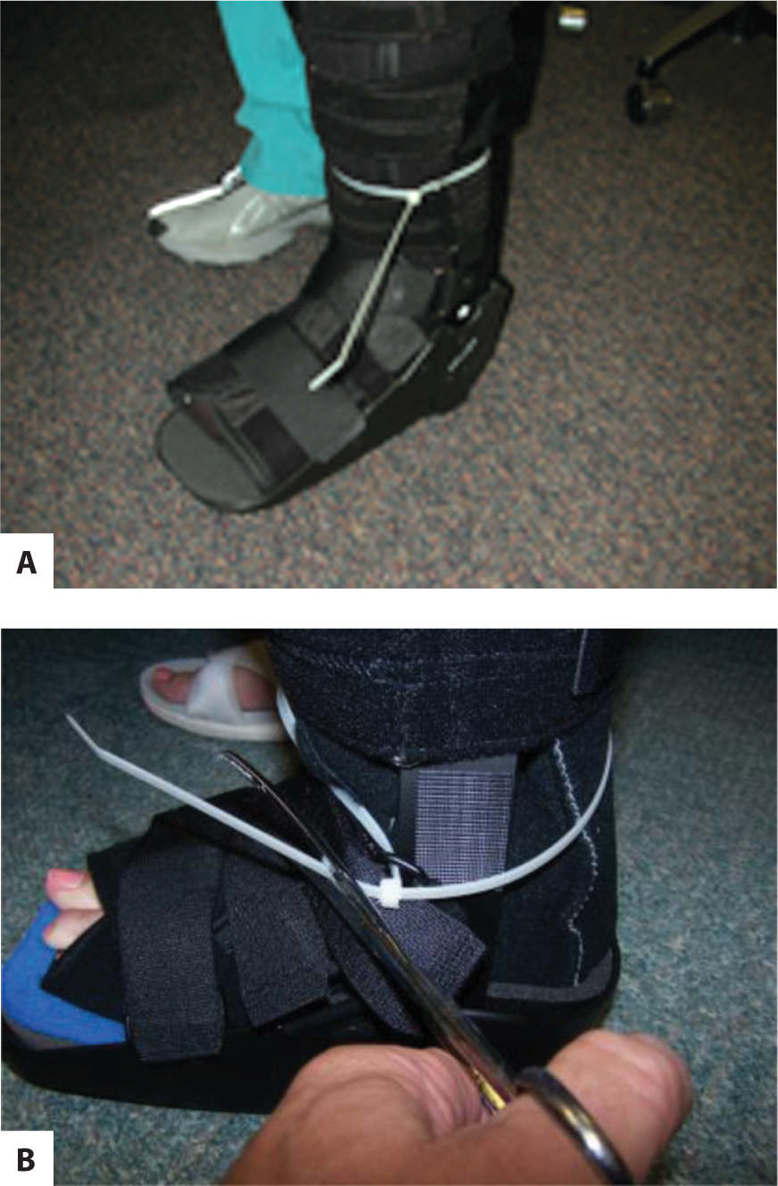
FIGURE 7-17 A and B. Instant total contact cast (iTCC) with ties to ensure compliance.
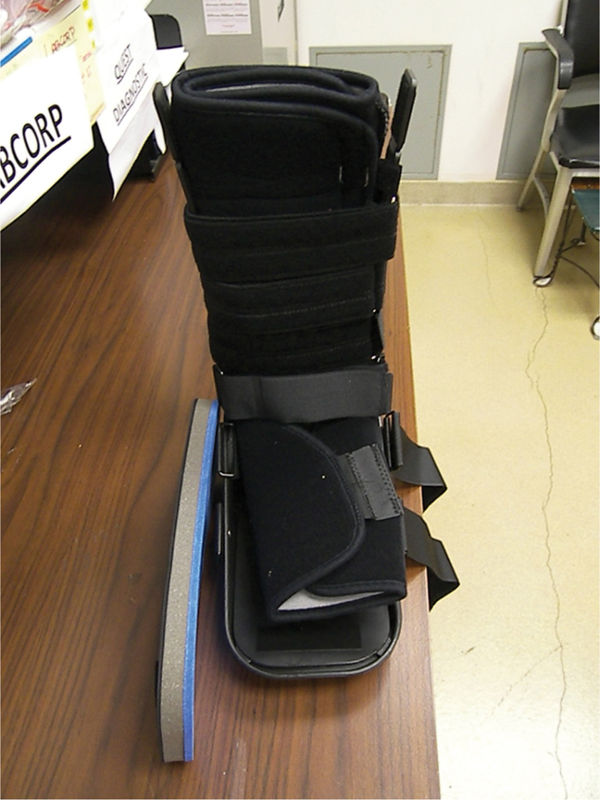
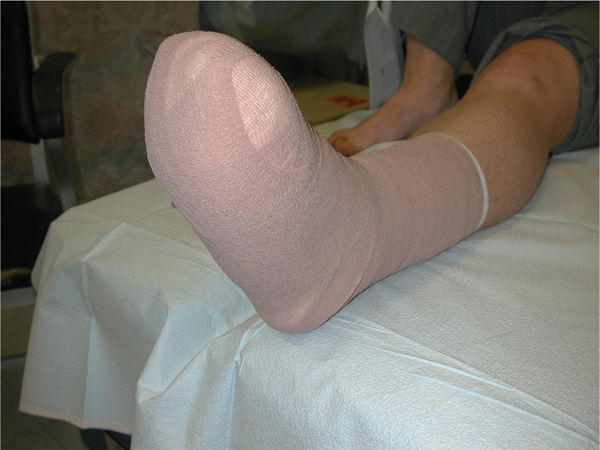
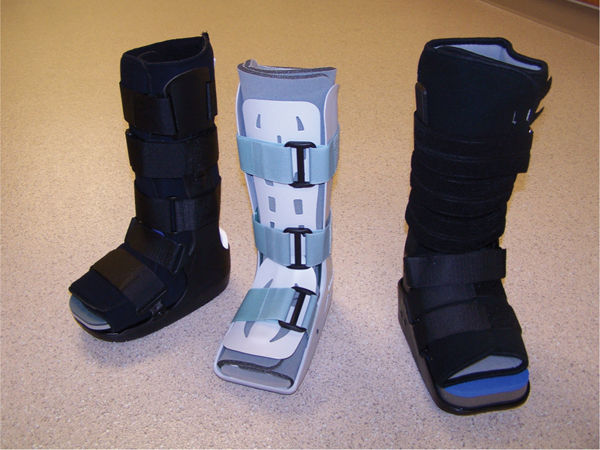
FIGURE 7-18 Removable cast walkers (RCWs) with insole.
FIGURE 7-19 The Rader football dressing.
FIGURE 7-20 The felted foam dressing.
FIGURE 7-21 Patellar tendon bearing ankle foot orthoses with rocker soled depth shoe Patellar tendon bearing (PTB) with ankle foot orthosis (AFO) can be combined with a rocker soled depth shoe, or in this case, with an extra wide shoe with molded insert. The toe deformity would determine if the athletic shoe is sufficient to prevent dorsal toe friction.
FIGURE 7-22 Limb-load removable cast walker.
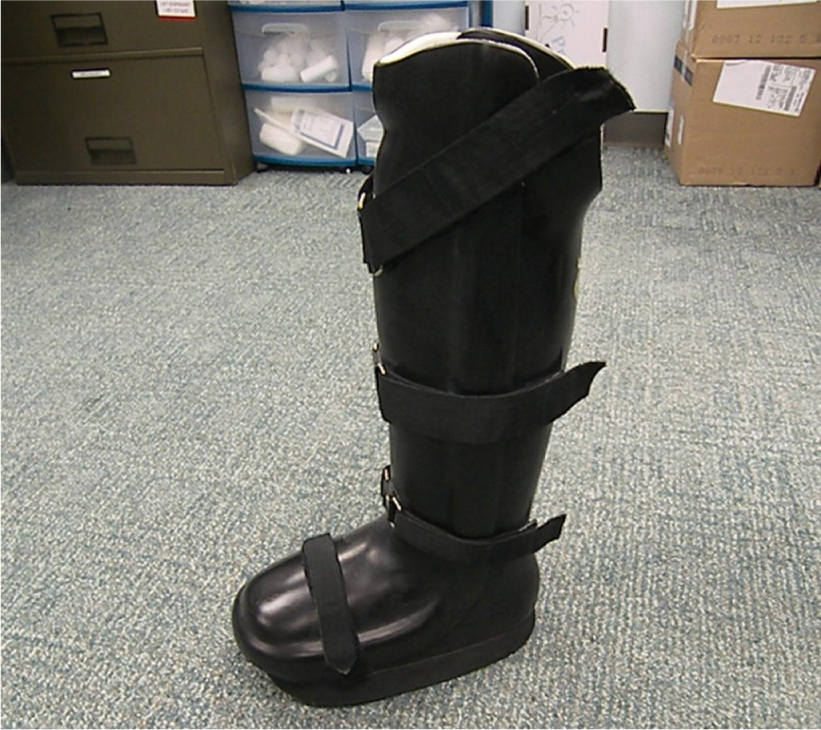
FIGURE 7-23 Charcot restraint orthopedic walker or CROW.
FIGURE 7-24 Modified Carville healing sandal—surgical shoe with a total contact heat-molded insert.
FIGURE 7-25 Commercial depth shoe with rocker sole and total contact insert.
FIGURE 7-26 Carville 22° rocker sole.
FIGURE 7-27 Custom molded shoe.
FIGURE 7-28 Silicone molded digital orthosis.
Eighty five per cent of all lower limb amputations in patients with diabetes are preceded by a foot ulcer.23–24 Those who develop a foot ulcer have a 55 times greater risk of infection24 and if a DFU is open for 30 days or longer that risk quadruples.25 The cost to the health care system is astronomical. Using conventional care, the average cost to obtain a healed ulcer is $56,000, due in part to the relative inefficiency of what is considered good wound care.26
In a recent meta-analysis, Margolis concluded that after 12 weeks of good wound care only 24.2% of ulcers were healed, and after an additional 8 weeks of the same care or 20 weeks, only 30.9% of those same wounds would have achieved complete closure.27
The total annual cost of diabetes treatment in 2002 (including direct and indirect costs) was estimated at $132 billion, or one out of every 10 health care dollars spent in the United States.28 A patient hospitalized for a DFU can expect a 59% longer length of stay than a diabetic patient hospitalized without a foot ulcer.29 A lower-extremity amputation (LEA) will be required by 14% to 24% of those DFU patients30 and unfortunately the incidence of LEA in people with diabetes continues to rise despite deliberate efforts to prevent amputations in the last decade.31
Biomechanical Changes with Diabetes
Diabetes has a particularly devastating effect on the form and function of the human foot. The human foot is an intricate ground interface for the human body providing sensory input, balance, and stability. The 26 bones, ligaments, and muscles are organized into a highly efficient mechanical system for maintaining balance and posture, sensing ground position and surface characteristics, and converting muscular and ground reactive forces into the various movements required to stand and move. The many nerves of the plantar surface provide constant feedback to the spinal cord and brain where an elaborate system of reflex and intentional movements keep the body’s center of gravity (COG) balanced between the two feet in the bipedal stance or over the weight-bearing foot in unilateral single support.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


 Describe the DM disease process and treatment options.
Describe the DM disease process and treatment options. Incorporate nutritional management into lifestyle.
Incorporate nutritional management into lifestyle. Incorporate physical activity into lifestyle.
Incorporate physical activity into lifestyle. Use medication(s) safely and for maximum therapeutic effectiveness.
Use medication(s) safely and for maximum therapeutic effectiveness. Monitor blood glucose and other parameters; interpret and use the results for self-management decision making.
Monitor blood glucose and other parameters; interpret and use the results for self-management decision making. Prevent, detect, and treat acute complications (ie, hyper and hypoglycemia).
Prevent, detect, and treat acute complications (ie, hyper and hypoglycemia). Prevent, detect, and treat chronic complications (eg, heart and kidney disease, retinopathy, Charcot foot disease).
Prevent, detect, and treat chronic complications (eg, heart and kidney disease, retinopathy, Charcot foot disease). Develop personal strategies to address psychosocial issues and concerns.
Develop personal strategies to address psychosocial issues and concerns. Develop personal strategies to promote health and behavior change.
Develop personal strategies to promote health and behavior change. Have you had a series of DM self-management classes?
Have you had a series of DM self-management classes? What medications do you take for DM? List the names and dose of each medication.
What medications do you take for DM? List the names and dose of each medication. How often do you monitor your blood sugars? What were they this morning?
How often do you monitor your blood sugars? What were they this morning? Do you take your diabetic medications regularly without fail?
Do you take your diabetic medications regularly without fail?