Cytokines: Introduction
|
The Concept of Cytokines
When cells and tissues in complex organisms need to communicate over distances greater than one cell diameter, soluble factors must be employed. A subset of these factors is most important when produced or released transiently under emergent conditions. When faced with an infection- or injury-related challenge, the host must orchestrate a complex and carefully choreographed series of steps. It must mobilize certain circulating white blood cells precisely to the relevant injured area (but not elsewhere) and guide other leukocytes involved in host defense, particularly T and B cells, to specialized lymphatic tissue remote from the infectious lesion but sufficiently close to contain antigens from the relevant pathogen. After a limited period of time in this setting (i.e., lymph node), antibodies produced by B cells and effector-memory T cells, can be released into the circulation and will localize at the site of infection. Soluble factors produced by resident tissue cells at the site of injury, by leukocytes and platelets that are recruited to the site of injury, and by memory T cells ultimately recruited to the area, all conspire to generate an evolving and effective response to a challenge to host defense. Most important, the level of this response must be appropriate to the challenge and the duration of the response must be transient; that is, long enough to decisively eliminate the pathogen, but short enough to minimize damage to healthy host tissues. Much of the cell-to-cell communication involved in the coordination of this response is accomplished by cytokines.
Cytokines (which include the large family of chemokines, discussed in Chapter 12) are soluble polypeptide mediators that play pivotal roles in communication between cells of the hematopoietic system and other cells in the body.1 Cytokines influence many aspects of leukocyte function including differentiation, growth, activation, and migration. While many cytokines are substantially upregulated in response to injury to allow a rapid and potent host response, cytokines also play important roles in the development of the immune system and in homeostatic control of the immune system under basal conditions. The growth and differentiation effects of cytokines are not limited to leukocytes, although we will not discuss soluble factors that principally mediate cell growth and differentiation of cells other than leukocytes in this chapter. The participation of cytokines in many parts of immune and inflammatory responses has prompted the examination of a variety of cytokines or cytokine antagonists (primarily antibodies and fusion proteins) as agents for pharmacologic manipulation of immune-mediated diseases. Only a few classes of effective cytokine drugs have emerged from the lengthy pathway of clinical trials to achieve FDA approval and widespread therapeutic use, but some of these drugs are now valuable therapeutics in dermatology. This chapter discusses these approved drugs and other promising biological agents still in clinical trials.
General features of cytokines are their pleiotropism and redundancy. Before the advent of a systematic nomenclature for cytokines, most newly identified cytokines were named according to the biologic assay that was being used to isolate and characterize the active molecule (e.g., T-cell growth factor for the molecule that was later renamed interleukin 2, or IL-2). Very often, independent groups studying quite disparate bioactivities isolated the same molecule that revealed the pleiotropic effects of these cytokines. For example, before being termed interleukin 1 (IL-1), this cytokine had been variously known as endogenous pyrogen, lymphocyte-activating factor, and leukocytic endogenous mediator. Many cytokines have a wide range of activities, causing multiple effects in responsive cells and a different set of effects in each type of cell capable of responding. The redundancy of cytokines typically means that in any single bioassay (such as induction of T-cell proliferation), multiple cytokines will display activity. In addition, the absence of a single cytokine (such as in mice with targeted mutations in cytokine genes) can often be largely or even completely compensated for by other cytokines with overlapping biologic effects.
Classifications of Cytokines
The first cytokines described had distinct and easily recognizable biological activities, exemplified by IL-1, IL-2, and the interferons (IFNs). The term cytokine was first coined by Cohen in 1975, to describe several such activities released into the supernatant of an epithelial cell line.2 Prior to this, such activities had been thought to be the exclusive domain of lymphocytes (lymphokines) and monocytes (monokines) and were considered a function of the immune system. Keratinocyte cytokines were first discovered in 1981,3 and the list of cytokines produced by this epithelial cell rivals nearly any other cell type in the body.4,5 The number of molecules that can be legitimately termed cytokines continues to expand and has brought under the cytokine rubric molecules with a broad range of distinct biological activities. The progress in genomic approaches has led to identification of novel cytokine genes based on homologies to known cytokine genes. Making sense of this plethora of mediators is more of a challenge than ever, and strategies to simplify the analysis of the cytokine universe are sorely needed.
A simple concept that continues to be extremely useful for discussion of cytokine function is the concept of “primary” and “secondary” cytokines.6 Primary cytokines are those cytokines that can, by themselves, initiate all the events required to bring about leukocyte infiltration in tissues. IL-1 (both α and β forms) and tumor necrosis factor (TNF; includes both TNF-α and TNF-β) function as primary cytokines, as do certain other cytokines that signal through receptors that trigger the nuclear factor κB (NF-κB) pathway. IL-1 and TNF are able to induce cell adhesion molecule expression on endothelial cells [selectins as well as immunoglobulin superfamily members such as intercellular adhesion molecule 1 (ICAM-1) and vascular cellular adhesion molecule 1 (VCAM-1)], to stimulate a variety of cells to produce a host of additional cytokines, and to induce expression of chemokines that provide a chemotactic gradient allowing the directed migration of specific leukocyte subsets into a site of inflammation (see Chapter 12). Primary cytokines can be viewed as part of the innate immune system (see Chapter 10), and in fact share signaling pathways with the so-called Toll-like receptors (TLRs), a family of receptors that recognize molecular patterns characteristically associated with microbial products.7 Although other cytokines sometimes have potent inflammatory activity, they do not duplicate this full repertoire of activities. Many qualify as secondary cytokines whose production is induced after stimulation by IL-1 and/or TNF family molecules. The term secondary does not imply that they are less important or less active than primary cytokines; rather, it indicates that their spectrum of activity is more restricted.
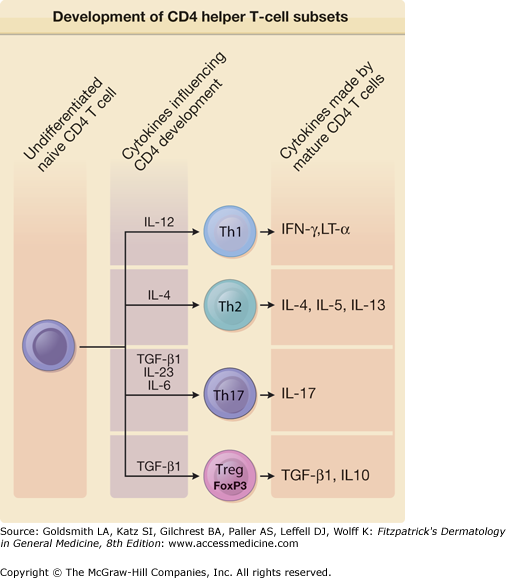
Another valuable concept that has withstood the test of time is the assignment of many T-cell-derived cytokines into groups based on the specific helper T-cell subsets that produce them (Fig. 11-1). The original two helper T-cell subsets were termed Th1 and Th2.8 Commitment to one of these two patterns of cytokine secretion also occurs with CD8 cytotoxic T cells and γ/δ T cells. Dominance of type 1 or type 2 cytokines in a T-cell immune response has profound consequences for the outcome of immune responses to certain pathogens and extrinsic proteins capable of serving as allergens. Over two decades after the original description of the Th1 and Th2 subsets, strong evidence has emerged that there are other functionally significant patterns of cytokine secretion by T cells. Most prominent among these newer T-cell lineages are Th17 cells and regulatory T cells (or Treg cells for short). The Th17 subset is distinguished by production of a high level of IL-17, but many Th17 cells also secrete IL-21 and IL-22. Th17 cells promote inflammation, and there is consistent evidence from human autoimmune diseases and mouse models of these diseases that IL-17-producing cells are critical effectors in autoimmune disease.9 A subset of T cells known as Treg cells has emerged as a crucial subset involved in the maintenance of peripheral self-tolerance.10 Two of the most distinctive features of Treg cells are their expression of the FoxP3 transcription factor and production of transforming growth factor-β (TGF-β), a cytokine that appears to be required for Treg cells to limit the excess activity of the proinflammatory T-cell subsets.11 IL-10 is also a significant contributor to the suppressive activity of Treg cells, particularly at some mucosal interfaces.12 Additional proposed helper T-cell subsets are follicular helper T cells (Tfh) that specialize in providing B cell help in germinal centers, Th9 cells distinguished by high levels of IL-9 production that function in antiparasite immunity along with Th2 cells, and Th22 cells associated with skin inflammation that produce Th22, but not other Th17-associated cytokines. Not only does each of these T-cell subsets exhibit distinctive patterns of cytokine production, cytokines are key factors in influencing the differentiation of naive T cells into these subsets. IL-12 is the key Th1-promoting factor, IL-4 is required for Th2 differentiation, and IL-6, IL-23, and TGF-β are involved in promoting Th17 development.
Figure 11-1
Cytokines control the development of specific CD4 helper T-cell subsets. The cytokine milieu at the time of activation of naive undifferentiated CD4 T cells has a profound influence on the ultimate pattern of cytokine secretion adopted by fully differentiated T cells. Subsets of effector CD4 T cells with defined patterns of cytokine secretion include T helper 1 (Th1), Th2, and Th17 cells. Regulatory CD4 T cells (Treg cells) express the FoxP3 transcription factor, and their effects are mediated in part by their production of transforming growth factor-β1 (TGF-β1) and/or interleukin 10 (IL-10). IFN = interferon; LT = lymphotoxin. (Adapted from Tato CM, O’shea JJ: What does it mean to be just 17? Nature 441:166, 2006.)
Not all useful classifications of cytokines are based solely on analysis of cytokine function. Structural biologists, aided by improved methods of generating homogenous preparations of proteins and establishment of new analytical methods (e.g., solution magnetic resonance spectroscopy) that complement the classical X-ray crystallography technique, have determined the three-dimensional structure of many cytokines. These efforts have led to the identification of groups of cytokines that fold to generate similar three-dimensional structures and bind to groups of cytokine receptors that also share similar structural features. For example, most of the cytokine ligands that bind to receptors of the hematopoietin cytokine receptor family are members of the four-helix bundle group of proteins. Four-helix bundle proteins have a shared tertiary architecture consisting of four antiparallel α-helical stretches separated by short connecting loops. The normal existence of some cytokines as oligomers rather than monomers was discovered in part as the result of structural investigations. For example, interferon-γ (IFN-γ) is a four-helix bundle cytokine that exists naturally as a noncovalent dimer. The bivalency of the dimer enables this ligand to bind and oligomerize two IFN-γ receptor complexes, thereby facilitating signal transduction. TNF-α and TNF-β are both trimers that are composed almost exclusively of β-sheets folded into a “jelly roll” structural motif. Ligand-induced trimerization of receptors in the TNF receptor family is involved in the initiation of signaling.
Signal Transduction Pathways Shared by Cytokines
To accomplish their effects, cytokines must first bind with specificity and high affinity to receptors on the cell surfaces of responding cells. Many aspects of the pleiotropism and redundancy manifested by cytokines can be understood through an appreciation of shared mechanisms of signal transduction mediated by cell surface receptors for cytokines. In the early years of the cytokine biology era, the emphasis of most investigative work was the purification and eventual cloning of new cytokines and a description of their functional capabilities, both in vitro and in vivo. Most of the cytokine receptors have now been cloned, and many of the signaling cascades initiated by cytokines have been described in great detail. The vast majority of cytokine receptors can be classified into a relatively small number of families and superfamilies (Table 11-1), the members of which function in an approximately similar fashion. Table 11-2 lists the cytokines of particular relevance for cutaneous biology, including the major sources, responsive cells, features of interest, and clinical relevance of each cytokine. Most cytokines send signals to cells through pathways that are very similar to those used by other cytokines binding to the same class of receptors. Individual cytokines often employ several downstream pathways of signal transduction, which accounts in part for the pleiotropic effects of these molecules. Nevertheless, we propose here that a few major signaling pathways account for most effects attributable to cytokines. Of particularly central importance are the NF-κB pathway and the Jak/STAT pathway, described in the following sections.
Receptor Family | Example | Major Signal Transduction Pathway(s) Leading to Biologic Effects |
|---|---|---|
IL-1 receptor family | IL-1R, type I | NF-κB activation via TRAF6 |
TNF receptor family | TNFR1 | NF-κB activation involving TRAF2 and TRAF5 |
Apoptosis induction via “death domain” proteins | ||
Hematopoietin receptor family (class I receptors) | IL-2R | Activation of Jak/STAT pathway |
IFN/IL-10 receptor family (class II receptors) | IFN-γR | Activation of Jak/STAT pathway |
Immunoglobulin superfamily | M-CSF R | Activation of intrinsic tyrosine kinase |
TGF-β receptor family | TGF-βR, types I and II | Activation of intrinsic serine/threonine kinase coupled to Smad proteins |
Chemokine receptor family | CCR5 | Seven transmembrane receptors coupled to G proteins |
Cytokine | Major Sources | Responsive Cells | Features of Interest | Clinical Relevance |
|---|---|---|---|---|
IL-1α | Epithelial cells | Infiltrating leukocytes | Active form stored in keratinocytes | IL-1Ra used to treat rheumatoid arthritis |
IL-1β | Myeloid cells | Infiltrating leukocytes | Caspase 1 cleavage required for activation | IL-1Ra used to treat rheumatoid arthritis |
IL-2 | Activated T cells | Activated T cells, Treg cells | Autocrine factor for activated T cells | IL-2 fusion toxin targets CTCL |
IL-4 | Activated Th2 cells, NKT cells | Lymphocytes, endothelial cells, keratinocytes | Causes B-cell class switching and Th2 differentiation | — |
IL-5 | Activated Th2 cells, mast cells | B cells, eosinophils | Regulates eosinophil response to parasites | Anti-IL-5 depletes eosinophils |
IL-6 | Activated myeloid cells, fibroblasts, endothelial cells | B cells, myeloid cells, hepatocytes | Triggers acute-phase response, promotes immunoglobulin synthesis | Anti-IL-6R used to treat rheumatoid arthritis |
IL-10 | T cells, NK cells | Myeloid and lymphoid cells | Inhibits innate and acquired immune responses | — |
IL-12 | Activated APCs | Th1 cells | Promotes Th1 differentiation, shares p40 subunit with IL-23 | Anti-p40 inhibits Crohn’s disease and psoriasis |
IL-13 | Activated Th2 cells, nuocytes | Monocytes, keratinocytes, endothelial cells | Mediates tissue responses to parasites | — |
IL-17 | Activated Th17 cells | Multiple cell types | Mediates autoimmune diseases | Potential drug target in autoimmune disease |
IL-22 | Activated Th17 cells and Th22 cells | Keratinocytes | Induces cytokines and antimicrobial peptides | Contributes to psoriasis |
IL-23 | Activated dendritic cells | Memory T cells, Th17 cells | Directs Th17 differentiation, mediates autoimmune disease | Anti-p40 inhibits Crohn’s disease and psoriasis |
IL-25 | Activated Th2 cells, mast cells | Th17 cells | Promotes Th2 differentiation, inhibits Th17 cells | — |
IL-27 | Activated APCs | Th1 cells | Promotes Th1 differentiation | — |
IL-35 | Treg cells | Th17 cells and Treg cells | Inhibits Th17 cells and expands Treg cells | — |
TNF-α | Activated myeloid, lymphoid, and epithelial cells | Infiltrating leukocytes | Mediates inflammation | Anti-TNF-α effective in psoriasis |
IFN-α and IFN-β | Plasmacytoid dendritic cells | Most cell types | Major part of innate antiviral response | Elicited by topical imiquimod application |
IFN-γ | Activated Th1 cells, CD8 T cells, NK cells, dendritic cells | Macrophages, dendritic cells, naive T cells | Macrophage activation, specific isotype switching | IFN-γ used to treat chronic granulomatous disease |
TSLP | Epithelial cells including keratinocytes | Dendritic cells, B cells, Th2 cells | Promotes Th2 differentiation | Involved in atopic diseases |
A major mechanism contributing to the extensive overlap between the biologic activities of the primary cytokines IL-1 and TNF is the shared use of the NF-κB signal transduction pathway. IL-1 and TNF use completely distinct cell surface receptor and proximal signaling pathways, but these pathways converge at the activation of the NF-κB transcription factor. NF-κB is of central importance in immune and inflammatory processes because a large number of genes that elicit or propagate inflammation have NF-κB recognition sites in their promoters.13 NF-κB-regulated genes include cytokines, chemokines, adhesion molecules, nitric oxide synthase, cyclooxygenase, and phospholipase A2.
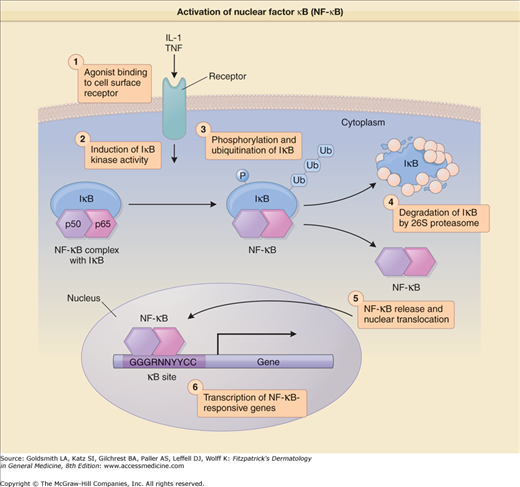
In unstimulated cells, NF-κB heterodimers formed from p65 and p50 subunits are inactive because they are sequestered in the cytoplasm as a result of tight binding to inhibitor proteins in the IκB family (Fig. 11-2). Signal transduction pathways that activate the NF-κB system do so through the activation of an IκB kinase (IKK) complex consisting of two kinase subunits (IKKα and IKKβ) and a regulatory subunit (IKKγ). The IKK complex phosphorylates IκBα and IκBβ on specific serine residues, yielding a target for recognition by an E3 ubiquitin ligase complex. The resulting polyubiquitination marks this IκB for rapid degradation by the 26S proteasome complex in the cytoplasm. Once IκB has been degraded, the free NF-κB (which contains a nuclear localization signal) is able to pass into the nucleus and induce expression of NF-κB-sensitive genes. The presence of κB recognition sites in cytokine promoters is very common. Among the genes regulated by NF-κB are IL-1β and TNF-α. This endows IL-1β and TNF-α with the capacity to establish a positive regulatory loop that favors persistent inflammation. Cytokines besides IL-1 and TNF that activate the NF-κB pathway as part of their signal transduction mechanisms include IL-17 and IL-18.
Figure 11-2
Activation of nuclear factor κB (NF-κB)-regulated genes after signaling by receptors for primary cytokines or by Toll-like receptors (TLRs) engaged by microbial products. Under resting conditions, NF-κB (a heterodimer of p50 and p65 subunits) is tightly bound to an inhibitor called IκB that sequesters NF-κB in the cytoplasm. Engagement of one of the TLRs or the signal transducing receptors for interleukin 1 (IL-1) or tumor necrosis factor (TNF) family members leads to induction of IκB kinase activity that phosphorylates IκB on critical serine residues. Phosphorylated IκB becomes a substrate for ubiquitination, which triggers degradation of IκB by the 26S proteasome. Loss of IκB results in release of NF-κB, which permits it to move to the nucleus and activate transcription of genes whose promoters contain κB recognition sites. Ub = ubiquitin.
Proinflammatory cytokines are not the only stimuli that can activate the NF-κB pathway. Bacterial products (e.g., lipopolysaccharide, or LPS), oxidants, activators of protein kinase C (e.g., phorbol esters), viruses, and ultraviolet (UV) radiation are other stimuli that can stimulate NF-κB activity. TLR4 is a cell surface receptor for the complex of LPS, LPS-binding protein, and CD14. The cytoplasmic domain of TLR4 is similar to that of the IL-1 receptor type 1 (IL-1R1) and other IL-1R family members and is known as the TIR domain (for Toll/IL-1 receptor).14 When ligand is bound to a TIR domain-containing receptor, one or more adapter proteins that also contain TIR domains are recruited to the complex. MyD88 was the first of these adapters to be identified; the other known adapters are TIRAP (TIR domain-containing adapter protein), TRIF (TIR domain-containing adapter inducing IFN-β), and TRAM (TRIF-related adapter molecule). Engagement of the adapter, in turn, activates one or more of the IL-1R-associated kinases (IRAK1 to IRAK4) that then signal through TRAF6, a member of the TRAF (TNF receptor-associated factor) family, and TAK1 (TGF-β-activated kinase) to activate the IKK complex.15
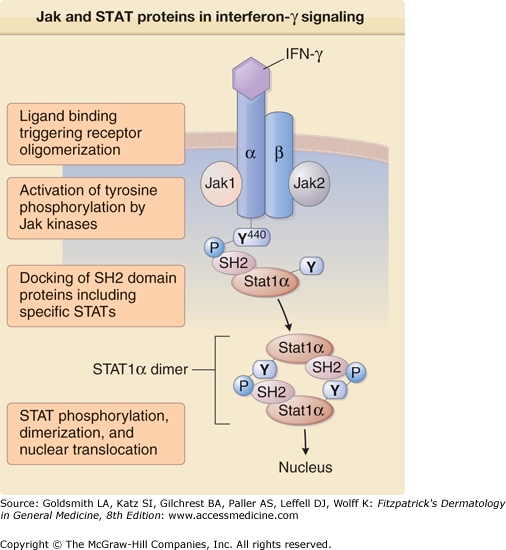
A major breakthrough in the analysis of cytokine-mediated signal transduction was the identification of a common cell surface to nucleus pathway used by the majority of cytokines. This Jak/STAT pathway was first elucidated through careful analysis of signaling initiated by IFN receptors (Fig. 11-3), but was subsequently shown to play a role in signaling by all cytokines that bind to members of the hematopoietin receptor family.16 The Jak/STAT pathway operates through the sequential action of a family of four nonreceptor tyrosine kinases (the Jaks or Janus family kinases) and a series of latent cytosolic transcription factors known as STATs (signal transducers and activators of transcription). The cytoplasmic portions of many cytokine receptor chains are noncovalently associated with one of the four Jaks [Jak1, Jak2, Jak3, and tyrosine kinase 2 (Tyk2)].
Figure 11-3
Participation of Jak (Janus kinase) and STAT (signal transducer and activator of transcription) proteins in interferon-γ (IFN-γ) signaling. Binding of human IFN-γ (a dimer) to its receptor brings about oligomerization of receptor complexes composed of α and β chains. The nonreceptor protein tyrosine kinases Jak1 and Jak2 are activated and phosphorylate critical tyrosine residues in the receptor such as the tyrosine at position 440 of the α chain (Y440). STAT1α molecules are recruited to the IFN-γ receptor based on the affinity of their Src homology 2 (SH2) domains for the phosphopeptide sequence around Y440. Receptor-associated STAT1α molecules then dimerize through reciprocal SH2-phosphotyrosine interactions. The resulting STAT1α dimers translocate to the nucleus and stimulate transcription of IFN-γ-regulated genes.
The activity of the Jak kinases is upregulated after stimulation of the cytokine receptor. Ligand binding to the cytokine receptors leads to the association of two or more distinct cytokine receptor subunits and brings the associated Jak kinases into close proximity with each other. This promotes cross-phosphorylation or autophosphorylation reactions that in turn fully activate the kinases. Tyrosines in the cytoplasmic tail of the cytokine receptor as well as tyrosines on other associated and newly recruited proteins are also phosphorylated. A subset of the newly phosphorylated tyrosines can then serve as docking points for attachment of additional signaling proteins bearing Src homology 2 (SH2) domains. Cytoplasmic STATs possess SH2 domains and are recruited to the phosphorylated cytokine receptors via this interaction. Homodimeric or heterodimeric STAT proteins are phosphorylated by the Jak kinases and subsequently translocate to the nucleus. In the nucleus they bind recognition sequences in DNA and stimulate transcription of specific genes, often in cooperation with other transcription factors. The same STAT molecules can be involved in signaling by multiple different cytokines. The specificity of the response in these instances may depend on the formation of complexes involving STATs and other transcription factors that then selectively act on a specific set of genes.
Interleukin 1 Family of Cytokines (Interleukins 1α, 1β, 18, 33)
IL-1 is the prototype of a cytokine that has been discovered many times in many different biologic assays. Distinct genes encode the α and β forms of human IL-1, with only 26% homology at the amino acid level. Both IL-1s are translated as 31-kDa molecules that lack a signal peptide, and both reside in the cytoplasm. This form of IL-1α is biologically active, but 31-kDa IL-1β must be cleaved by caspase 1 (initially termed interleukin-1β-converting enzyme) in a multiprotein cytoplasmic complex called the inflammasome to generate an active molecule.17
In general, IL-1β appears to be the dominant form of IL-1 produced by monocytes, macrophages, Langerhans cells, and dendritic cells, whereas IL-1α predominates in epithelial cells, including keratinocytes. This is likely to relate to the fact that epithelial IL-1α is stored in the cytoplasm of cells that comprise an interface with the external environment. Such cells, when injured, release biologically active 31-kDa IL-1α and, by doing so, can initiate inflammation.6 However, if uninjured, these cells will differentiate and ultimately release their IL-1 contents into the environment. Leukocytes, including dendritic and Langerhans cells, carry their cargo of IL-1 inside the body, where its unregulated release could cause significant tissue damage. Thus, biologically active IL-1β release from cells is controlled at several levels: IL-1β gene transcription, caspase 1 gene transcription, and availability of the adapter proteins that interact with caspase 1 in the inflammasome to allow the generation of mature IL-1β. IL-1β stimulates the egress of Langerhans cells from the epidermis during the initiation of contact hypersensitivity, a pivotal event that leads to accumulation of Langerhans cells in skin-draining lymph nodes. Studies of mice deficient in IL-1α and IL-1β genes suggest that both molecules are important in contact hypersensitivity, but that IL-1α is more critical.
Active forms of IL-1 bind to the IL-1R1 or type 1 IL-1 receptor.14 This is the sole signal-transducing receptor for IL-1, and its cytoplasmic domain has little homology with other cytokine receptors, showing greatest homology with the Toll gene product identified in Drosophila. A second cell surface protein, the IL-1R accessory protein, or IL-1RAcP, must associate with IL-1R1 for signaling to occur. When IL-1 engages the IL-1R1/IL-1RAcP complex, recruitment of the MyD88 adapter occurs, followed by interactions with one or more of the IRAKs. These kinases in turn associate with TRAF6. Stepwise activation and recruitment of additional signaling molecules culminate in the induction of IKK activity. The net result is the activation of a series of NF-κB-regulated genes.
A molecule known as the IL-1 receptor antagonist
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree