Cutaneous Reactions to Drugs: Introduction
|
Complications of drug therapy are a major cause of patient morbidity and account for a significant number of patient deaths.1 Drug eruptions range from common nuisance eruptions to rare or life-threatening drug-induced diseases. Drug reactions may be solely limited to the skin, or they may be part of a systemic reaction, such as drug hypersensitivity syndrome or toxic epidermal necrolysis (TEN) (see Chapter 40).
Epidemiology
A systematic review of the medical literature, encompassing nine studies, concluded that cutaneous reaction rates varied from 0% to 8% and were highest for antibiotics.2 Outpatient studies of cutaneous adverse drug reactions (ADRs) estimate that 2.5% of children who are treated with a drug, and up to 12% of children treated with an antibiotic, will experience a cutaneous reaction.3
Etiology
In the evaluation of a patient with a history of a suspected ADR, it is important to obtain a detailed medication history, including use of over-the-counter preparations and herbal and naturopathic remedies. New drugs started within the preceding 3 months, especially those within 6 weeks, are potential causative agents for most cutaneous eruptions (exceptions include drug-induced lupus, drug-induced pemphigus, and drug-induced cutaneous pseudolymphoma), as are drugs that have been used intermittently.
Pathogenesis of Drug Eruptions
Constitutional factors influencing the risk of cutaneous eruption include pharmacogenetic variation in drug-metabolizing enzymes and human leukocyte antigen (HLA) associations. Acetylator phenotype alters the risk of developing drug-induced lupus due to hydralazine, procainamide, and isoniazid. HLA-DR4 is significantly more common in individuals with hydralazine-related drug-induced lupus than in those with idiopathic systemic lupus erythematosus.4 HLA factors may also influence the risk of reactions to nevirapine, abacavir, carbamazepine, and allopurinol.5–7
Many drugs associated with severe idiosyncratic drug reactions are metabolized by the body to form reactive, or toxic, drug products.8 These reactive products comprise only a small proportion of a drug’s metabolites and are usually rapidly detoxified. However, patients with drug hypersensitivity syndrome, TEN, and Stevens–Johnson syndrome (SJS) resulting from treatment with sulfonamide antibiotics and the aromatic anticonvulsants (e.g., carbamazepine, phenytoin, phenobarbital, primidone, and oxcarbazepine) show greater sensitivity in in vitro assessments to the oxidative, reactive metabolites of these drugs than do control subjects.9
Acquired factors also alter an individual’s risk of drug eruption. Active viral infection and concurrent use of other medications have been shown to alter the frequency of drug-associated eruptions. Reactivation of latent viral infection with human herpes virus 6 also appears common in drug hypersensitivity syndrome and may be partially responsible for some of the clinical features and/or course of the disease.10,11 Viral infections may act as, or generate the production of, danger signals that lead to damaging immune responses to drugs, rather than immune tolerance.
Drug–drug interactions may also alter the risk of cutaneous eruption. Valproic acid increases the risk of severe cutaneous adverse reactions to lamotrigine, another anticonvulsant.12 The basis of these interactions and reactions is unknown, but they may represent a combination of factors, including alterations in drug metabolism, drug detoxification, antioxidant defenses, and immune reactivity.
The course and outcome of drug-induced disease are also influenced by host factors. Older age may delay the onset of drug eruptions and has been associated with a higher mortality rate in some severe reactions. A higher mortality rate is also observed in patients with severe reactions who have underlying malignancy.13 The pathogenesis of most drug eruptions is not understood, although the clinical features of most drug reactions are consistent with immune-mediated disease. The immune system may target the native drug, its metabolic products, altered self, or a combination of these factors.14
Morphologic Approach to Drug Eruptions
Although there are many presentations of cutaneous drug eruptions, the morphology of many cutaneous eruptions may be exanthematous, urticarial, blistering, or pustular. The extent of the reaction is variable. For example, once the morphology of the reaction has been documented, a specific diagnosis [e.g., fixed drug eruption (FDE) or acute generalized exanthematous pustulosis (AGEP)] can be made. The reaction may also present as a systemic syndrome [e.g., serum sickness-like reaction or hypersensitivity syndrome reaction (HSR)]. Fever is generally associated with such systemic cutaneous ADRs.
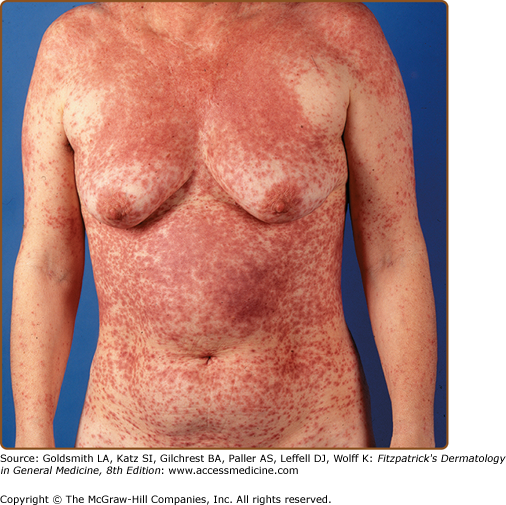
Exanthematous eruptions, sometimes referred to as morbilliform or maculopapular, are the most common form of drug eruptions, accounting for approximately 95% of skin reactions2 (Fig. 41-1). Simple exanthems are erythematous changes in the skin without evidence of blistering or pustulation. The eruption typically starts on the trunk and spreads peripherally in a symmetric fashion. Pruritus is almost always present. These eruptions usually occur within 1 week of initiation of therapy and may appear 1 or 2 days after drug therapy has been discontinued.15 Resolution, usually with 7–14 days, occurs with a change in color from bright red to a brownish red, which may be followed by desquamation. The differential diagnosis in these patients includes an infectious exanthem (e.g., viral, bacterial, or rickettsial), collagen vascular disease, and infections.
Exanthematous eruptions can be caused by many drugs, including β-lactams (“the penicillins”), sulfonamide antimicrobials, nonnucleoside reverse transcriptase inhibitors (e.g., nevirapine), and antiepileptic medications. Studies have shown that drug-specific T cells play a major role in exanthematous, bullous, and pustular drug reactions.16 In patients who have concomitant infectious mononucleosis, the risk of developing an exanthematous eruption while being treated with an aminopenicillin (e.g., ampicillin) increases from 3%–7% to 60%-100%.17 A similar drug–viral interaction has been observed in 50% of patients infected with human immunodeficiency virus (HIV) who are exposed to sulfonamide antibiotics.14
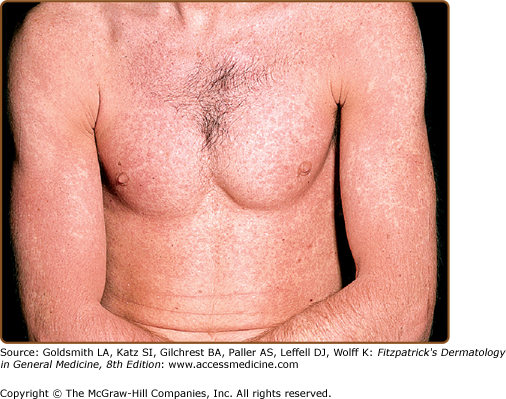
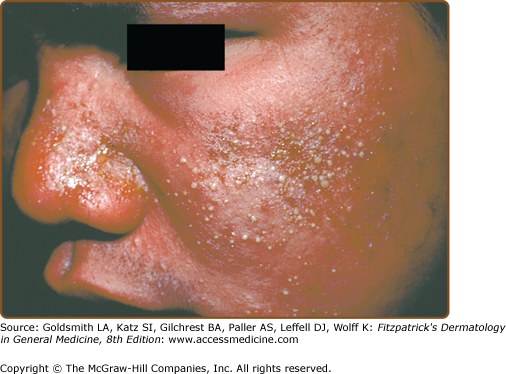
An exanthematous eruption in conjunction with fever and internal organ inflammation (e.g., liver, kidney, central nervous system) signifies a more serious reaction, known as the hypersensitivity syndrome reaction, drug-induced hypersensitivity reaction (DIHS) or drug reaction with eosinophilia and systemic symptoms (DRESS) (Table 41-1). It occurs in approximately 1 in 3,000 exposures to agents such as aromatic anticonvulsants, lamotrigine, sulfonamide antimicrobials, dapsone, nitrofurantoin, nevirapine, minocycline, metronidazole, and allopurinol (Fig. 41-2). HSR occurs most frequently on first exposure to the drug, with initial symptoms starting 1–6 weeks after exposure. Fever and malaise are often the presenting symptoms. Atypical lymphocytosis with subsequent eosinophilia may occur during the initial phases of the reaction in some patients. Although most patients have an exanthematous eruption, more serious cutaneous manifestations may be evident (Fig. 41-3). Internal organ involvement can be asymptomatic.11 Some patients may become hypothyroid due to an autoimmune thyroiditis approximately 2 months after the first symptoms appear.18
Clinical Presentation | Drug Eruption | Fever | Internal Organ Involvement | Arthralgia | Lymphadenopathy | Implicated Drugs |
|---|---|---|---|---|---|---|
Hypersensitivity syndrome reaction | Exanthem, exfoliative dermatitis, pustular eruptions, SJS/TEN | Present | Present | Absent | Present | Aromatic anticonvulsants (e.g., phenytoin, phenobarbital, carbamazepine), sulfonamide antibiotics, dapsone, minocycline, allopurinol, lamotrigine |
Serum sickness-like reaction | Urticaria, exanthem | Present | Absent | Present | Present | Cefaclor, cefprozil, bupropion, minocycline, infliximab, rituximab |
Drug-induced lupus | Usually absent | Present/absent | Present/absent | Present | Absent | Procainamide, hydralazine, isoniazid, minocycline, acebutolol |
Drug-induced subacute cutaneous lupus erythematosus | Papulosquamous or annular cutaneous lesion (often photosensitive) | Absent | Absent | Absent | Absent | Thiazide diuretics, calcium channel blockers, ACE inhibitors |
Acute generalized exanthematous pustulosis | Nonfollicular pustules on an edematous erythematous base | Present | Absent | Absent | Absent | β Blockers, macrolide antibiotics, calcium channel blockers |
The formation of toxic metabolites of the aromatic anticonvulsants may play a pivotal role in the development of HSR.9 In most individuals, the chemically reactive metabolites that are produced are detoxified by epoxide hydroxylases. However, if detoxification is defective, one of the metabolites may act as a hapten and initiate an immune response, stimulate apoptosis, or cause cell necrosis directly. Approximately 70%–75% of patients who develop anticonvulsant HSR in response to one aromatic anticonvulsant show cross-reactivity to the other aromatic anticonvulsants. In addition, in vitro testing shows that there is a pattern of inheritance of HSR induced by anticonvulsants. Thus, counseling of family members and disclosure of risk are essential.
Sulfonamide antimicrobials are both sulfonamides (contain SO2-NH2) and aromatic amines (contain a benzene ring-NH2). Aromatic amines can be metabolized to toxic metabolites, namely, hydroxylamines and nitroso compounds.19 In most people, the metabolite is detoxified. However, HSRs may occur in patients who either form excess oxidative metabolites or are unable to detoxify such metabolite. Because siblings and other first-degree relatives may be at an increased risk (perhaps as high as 1 in 4) of developing a similar adverse reaction, counseling of family members is essential.
Other aromatic amine-containing drugs, such as procainamide, dapsone, and acebutolol, may also be metabolized to chemically reactive compounds. It is recommended that patients who develop symptoms compatible with a sulfonamide-induced HSR avoid these aromatic amines, because the potential exists for cross-reactivity. However, cross-reactivity is much less likely to occur between sulfonamides antimicrobials and drugs that are not aromatic amines (e.g., sulfonylureas, thiazide diuretics, furosemide, celecoxib, and acetazolamide).20
Allopurinol is associated with the development of serious drug reactions, including HSR. Active infection or reactivation of HHV-6 has been observed in patients who develop allopurinol HSR.21 Allopurinol-induced severe adverse reactions, specifically HSR and SJS/TEN spectrum, have been strongly associated with a genetic predisposition in Han Chinese and Thai populations; presence of the HLA-B*5801 allele was found to be an important genetic risk factor.6,22
Urticaria is characterized by pruritic red wheals of various sizes. Individual lesions generally last for less than 24 hours, although new lesions can commonly develop. When deep dermal and subcutaneous tissues are also swollen, the reaction is known as angioedema. Angioedema is frequently unilateral and nonpruritic and lasts for 1–2 hours, although it may persist for 2–5 days.21
Urticaria and angioedema, when associated with drug use, are usually indicative of an immunoglobulin (Ig) E-mediated immediate hypersensitivity reaction. This mechanism is typified by immediate reactions to penicillin and other antibiotics (see Chapter 38). Signs and symptoms of IgE-mediated allergic reactions typically include pruritus, urticaria, cutaneous flushing, angioedema, nausea, vomiting, diarrhea, abdominal pain, nasal congestion, rhinorrhea, laryngeal edema, and bronchospasm or hypotension. Urticaria and angioedema can also be caused by non-IgE-mediated reactions that result in direct and nonspecific liberation of histamine or other mediators of inflammation.15 Drug-induced non-IgE-mediated urticaria and angioedema are usually related to nonsteroidal anti-inflammatory drugs (NSAIDs), angiotensin converting enzyme (ACE)-inhibitors and opioids.
Serum sickness-like reactions (see Table 41-1) are defined by the presence of fever, rash (usually urticarial), and arthralgias 1–3 weeks after initiation of drug therapy. Lymphadenopathy and eosinophilia may also be present; however, in contrast to true serum sickness, immune complexes, hypocomplementemia, vasculitis, and renal lesions are absent.
Cefaclor is associated with an increased relative risk of serum sickness-like reactions. The overall incidence of cefaclor-induced serum sickness-like reactions has been estimated to be 0.024%–0.2% per course of cefaclor prescribed. In genetically susceptible hosts, a reactive metabolite is generated during the metabolism of cefaclor that may bind with tissue proteins and elicit an inflammatory response manifesting as a serum sickness-like reaction.23
Other drugs that have been implicated in serum sickness-like reactions are cefprozil, bupropion, minocycline, and rituximab24 as well as infliximab.25 The incidence of serum sickness-like reactions caused by these drugs is unknown.
Acneiform eruptions are associated with the use of iodides, bromides, adrenocorticotropic hormone, glucocorticoids, isoniazid, androgens, lithium, actinomycin D, and phenytoin. Drug-induced acne may appear in atypical areas, such as on the arms and legs, and is most often monomorphous. Comedones are usually absent. The fact that acneiform eruptions do not affect prepubertal children indicates that previous hormonal priming is a necessary prerequisite. In cases in which the offending agent cannot be discontinued, topical tretinoin may be useful.26 An acneiform eruption often occurs during treatment with epidermal growth factor receptor inhibitors (e.g., gefitinib, erlotinib, cetuximab). The acneiform rash is often accompanied by paronychia, dry skin, and skin fissures. The eruption is dose dependent, with respect to both incidence and severity.27 In a systemic review and meta-analysis encompassing over 1,000 patients receiving cetuximab as a single-agent, the incidence of an acneiform eruption was 81.6%.28
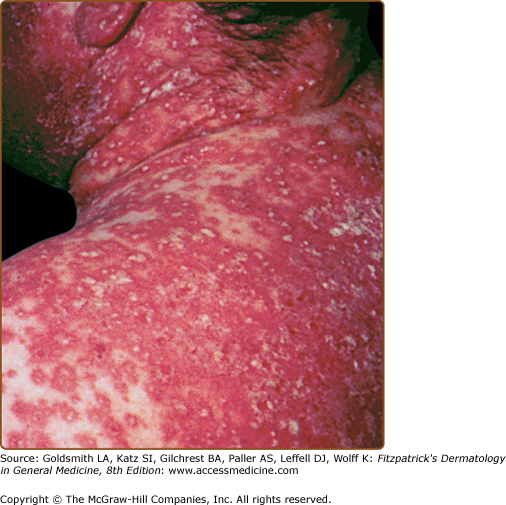
AGEP is an acute febrile eruption that is often associated with leukocytosis (Fig. 41-4 and Table 41-1). After initiation of the implicated drug, 1–3 weeks may elapse before skin lesions appear. The lesions often start on the face or major skin creases. Generalized desquamation occurs approximately 2 weeks later. The estimated incidence of AGEP is approximately 1–5 cases per million per year. AGEP is most commonly associated with β-lactam and macrolide antibiotics, anticonvulsants, and calcium channel blockers.29 Differential diagnosis includes pustular psoriasis, HSR with pustulation, subcorneal pustular dermatosis (Sneddon–Wilkinson disease), pustular vasculitis, or in severe cases of AGEP, TEN. The typical histopathologic analysis of AGEP lesions shows spongiform subcorneal and/or intraepidermal pustules, an often marked edema of the papillary dermis, and perivascular infiltrates with neutrophils and exocytosis of some eosinophils. Discontinuance of therapy is usually the extent of treatment necessary in most patients, although some patients may require the use of corticosteroids. Patch tests have been used in the diagnosis of AGEP.30