93
Cutaneous Melanoma
• Some cutaneous melanomas (CMs) arise de novo, whereas others arise within precursor lesions (e.g. melanocytic nevi; see Chapter 92).
• Tremendous advances have been made in understanding the molecular pathways and mutations from which many CMs originate, resulting in identification of genetic markers, tools for aiding in the histologic diagnosis, and targeted treatments for CM (Figs. 93.1 and 93.2; see Table 93.11).
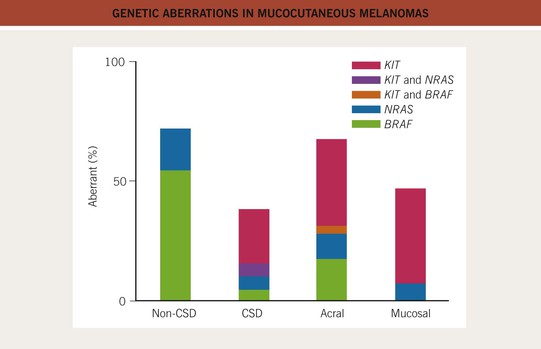
Fig. 93.1 Genetic aberrations in mucocutaneous melanomas. CSD, skin with chronic sun-induced changes such as marked solar elastosis; non-CSD, skin without chronic sun-induced damage. From Curtin JA, Busam K, Pinkel D, Bastian BC. Somatic activation of KIT in distinct subtypes of melanoma. J. Clin. Oncol. 2006;24:4340–4346.
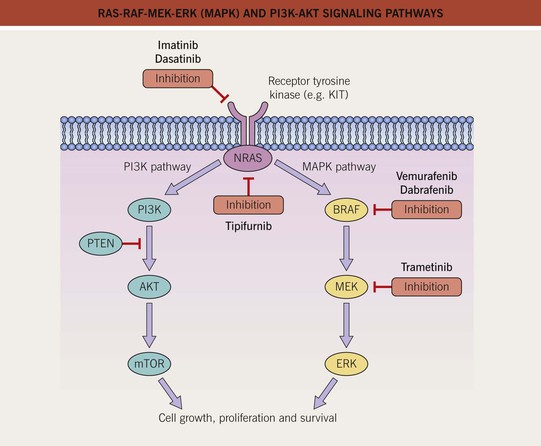
Fig. 93.2 RAS-RAF-MEK-ERK (MAPK) and PI3K-AKT signaling pathways. The mitogen-activated protein kinase (MAPK) pathway is physiologically activated by growth factor binding to receptor tyrosine kinases. The stimulus is relayed to the nucleus via the GTPase activity of NRAS and the kinase activity of BRAF, MEK, and ERK. Within the nucleus, this results in increased transcription of genes involved in cell growth, proliferation and survival. The central role of this pathway in melanocytic neoplasia is highlighted by the fact that either NRAS or BRAF is mutated in approximately 80% of all cutaneous melanomas and melanocytic nevi. The PI3K-AKT pathway is another important regulator of cell survival, growth, and apoptosis. A key inhibitor of this pathway is PTEN, and inactivation of the gene that encodes PTEN via mutations, deletions, or promoter methylation also occurs in cutaneous melanomas. As a result, there is increased activity of the PI3K-AKT signaling pathway. PI3K, phosphoinositide 3-kinase; PTEN, phosphatase and tensin homolog. Adapted from Eggermont AM, Robert C. Melanoma in 2011: A new paradigm tumor for drug development. Nat. Rev. Clin. Oncol. 2012;9:74–76.
Epidemiology
• There has been a marked increase in the incidence of CM during the past 40–50 years, with a modest increase in mortality, especially among older males (Figs. 93.3 and 93.4).
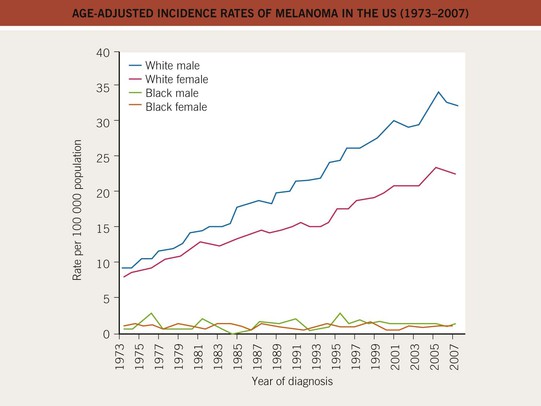
Fig. 93.3 Age-adjusted incidence rates of melanoma in the United States (1973–2007). The incidence has increased in the male and female Caucasian populations during the past three decades, and this trend has not yet stopped. The African-American population shows a constant low incidence, mostly caused by acral and mucosal melanomas. Data from the Surveillance, Epidemiology, and End Results (SEER) program of the National Cancer Institute.
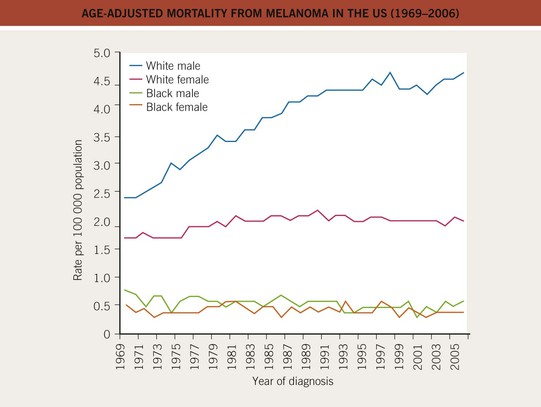
Fig. 93.4 Age-adjusted mortality from melanoma in the United States (1969–2006). The mortality has increased, especially for the male Caucasian population, during the past three decades. For females, the increase was less pronounced and has leveled off since the 1980s. Data from the Surveillance, Epidemiology, and End Results (SEER) program of the National Cancer Institute.
• Death occurs at a younger age than for most other cancers.
• Risk factors are divided into three major groups: genetic, environmental, and phenotypic (Table 93.1).
• Primary prevention: sun protective measures and avoidance of tanning beds.
Clinical
• The clinical presentation of CM varies greatly; although the ABCDE‘s (Asymmetry, Border irregularity, Color variegation, Diameter >6 mm, Evolution) are often used in public awareness campaigns to help promote the clinical recognition of CM, they have their shortcomings; to rectify this, the EFG rule (Elevated, Firm, Growing) has been added for nodular and amelanotic CMs.
• There are four major subtypes of primary invasive CM, based on clinicopathologic features:
– Superficial spreading melanoma (SSM; ~60% of melanomas) (Fig. 93.5).
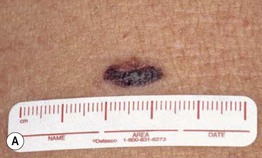
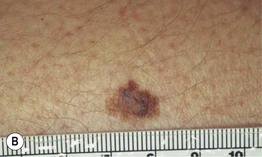
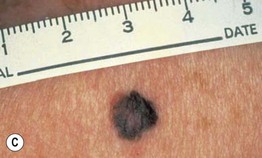
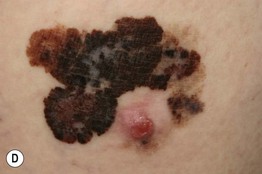
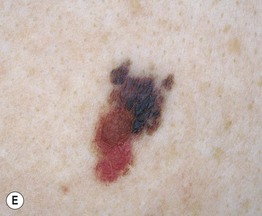
Fig. 93.5 Superficial spreading melanomas. A–C All of these early lesions demonstrate asymmetry due to variation in color and irregularity in outline. In addition, there is pink discoloration in (C). A, B were less than 0.5 mm in thickness and (C) was 0.8 mm. D In this more advanced lesion, note the asymmetry, irregular borders, variation in colors, scarlike regression zones, and an inferior pink papule, indicating vertical growth phase. E Superficial spreading melanoma arising within a compound melanocytic nevus. Note the irregular outline and variable pigmentation. A, Courtesy, Kalman Watsky, MD; B, C, Courtesy, Jean L. Bolognia, MD; D, Courtesy, Claus Garbe, MD.
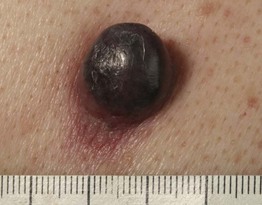
– Nodular melanoma (NM; ~20%) (Fig. 93.6).
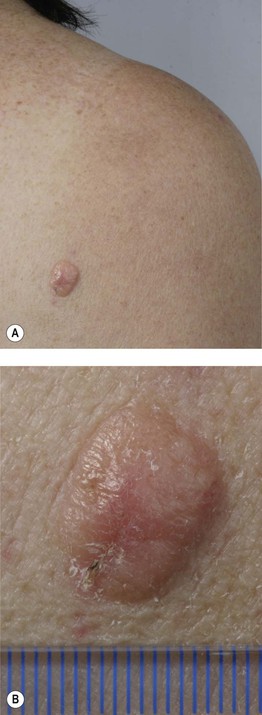
– Lentigo maligna melanoma (LMM; ~9%) (Fig. 93.7).
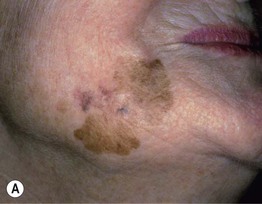
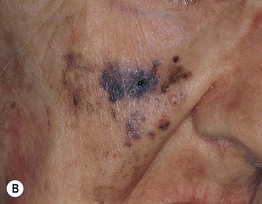
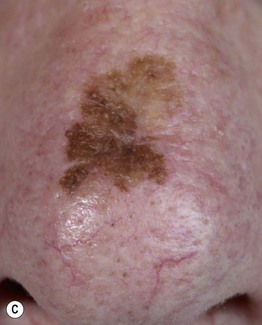
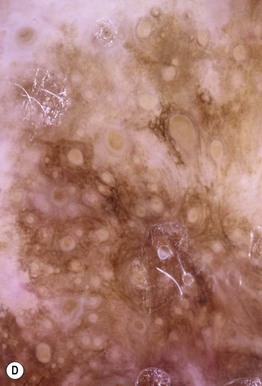
Fig. 93.7 Lentigo maligna (LM) and lentigo maligna melanoma (LMM). A Large-sized brown patch with some variation in color representing lentigo maligna. B Similarly sized lesion, but with marked variation in color and topography. There was invasion to a depth of 1.1 mm within the blue-gray papules, representing LMM. C LMM on the dorsal nose, with irregular borders, light to dark brown color, and marked asymmetry. D Dermoscopy of this lesion demonstrating annular structures corresponding to follicular openings surrounded by melanoma cells (‘circle in a circle’). B, Courtesy, Kalman Watsky, MD; C, D, Courtesy, Claus Garbe, MD.
– Acral lentiginous melanoma (ALM; ~4%) (Fig. 93.8).
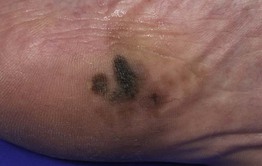
Fig. 93.8 Acral lentiginous melanoma. Irregularly pigmented lesion on the plantar surface of the foot. These tumors may become amelanotic and are then easily overlooked or misdiagnosed as verrucae or other benign lesions, often resulting in a significantly delayed diagnosis. Courtesy, Claus Garbe, MD.
• In cutaneous melanoma in situ (MIS), the malignancy is confined to the epidermis and/or the hair follicle epithelium (Fig. 93.9); all types of CM (except for NM) can have an in situ phase; lentigo maligna is a type of MIS that arises within chronically sun-damaged skin and can remain in situ for years to decades.
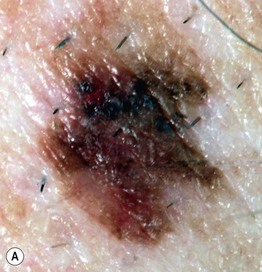
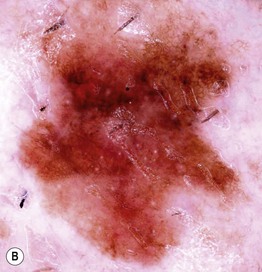
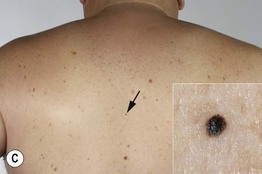
Fig. 93.9 Melanoma in situ. A Note the asymmetry, border irregularity, color variation, and erythema (‘little red riding hood’ sign). B The dermoscopic findings include a broadened network, dots, and some globules. C This melanoma in situ measured less than 3 mm in diameter. Small melanomas can be easily overlooked, especially if they are less heavily pigmented. This lesion was the ‘ugly duckling’ of all of this patient’s nevi. Dermoscopy assists in making an earlier diagnosis. Courtesy, Claus Garbe, MD.
– Amelanotic melanoma: color ranges from pink to red and any type of CM may be amelanotic (Fig. 93.10); children can develop amelanotic nodular CM, and the lesion may resemble a persistent arthropod bite or pyogenic granuloma.