Chapter 33 Critical care in the severely burned
Organ support and management of complications
![]() Access the complete reference list online at http://www.expertconsult.com
Access the complete reference list online at http://www.expertconsult.com
![]() IN THIS CHAPTER
IN THIS CHAPTER ![]() PowerPoint Presentation Online
PowerPoint Presentation Online
Introduction
Over 500 000 people are burned and seek medical care in the United States every year; most of these have minor injuries and are treated in the outpatient setting. However, approximately 50 000 burns per year (10%) are moderate to severe and require hospitalization for treatment. Some of these will require critical care for at least part of their hospitalization, and some for months. About 4000 die each year from complications related to the burn.1,2 Burn deaths generally occur in a bimodal distribution, either immediately after the injury, or weeks later due to multiple organ failure, a pattern similar to all injury-related deaths.
Morbidity and mortality from burns are decreasing in incidence. Recent reports revealed a 50% decline in burn-related deaths and hospital admissions in the USA over the last 20 years.2 The declines were likely most affected by prevention efforts, with fewer potentially fatal burns, and improved critical care and wound management for those who still sustain the injury. In 1949, Bull and Fisher reported 50% mortality rates for children aged 0–14 years with burns of 49% of total body surface area (TBSA), 46% TBSA for patients aged 15–44, 27% TBSA for those aged between 45 and 64, and 10% TBSA for those 65 and older.3 These dismal statistics have improved, with the latest studies reporting a 50% mortality for >95% TBSA burns in children 14 years and under, 75% TBSA burns in adults, and around 30% TBSA in the elderly.4 Therefore, a healthy young patient with almost any size burn should be expected to live, whereas the prospects for those who are older are certainly improving with modern wound treatment and critical care techniques.
Burned patients generally die from one of two causes: ‘burn shock’ and immolation resulting in early deaths, and multiple organ failure and sepsis leading to late deaths. With the advent of vigorous fluid resuscitation protocols in the severely burned, irreversible burn shock has been replaced by sepsis and subsequent multiple organ failure as the leading cause of death associated with burns in those who do not die at the scene by a margin of 2 to 1.4 Those with some risk of mortality who do not die precipitously will be treated by what is termed critical care, a service performed in specialized units containing the equipment, supplies, and personnel to institute intensive monitoring and life-sustaining organ support to promote recovery.
Critical illness in burned patients is most commonly associated with sepsis. In a pediatric burn population with massive burns > 80% TBSA, 17.5% of the children developed sepsis, defined as bacteraemia with clinical signs of infection.5 Mortality in the whole group was 33%, most of whom succumbed to multiple organ failure. Some were bacteremic and ‘septic,’ but the majority were not. These findings highlight the observation that the development of severe critical illness and multiple organ failure is often associated with infection, but it is by no means required to develop this syndrome. What is required is an inflammatory focus, which in severe burns is the massive skin injury that requires inflammation to heal.
It is postulated that progression of patients to multiple organ failure exists in a continuum with the systemic inflammatory response syndrome (SIRS).6 Nearly all burn patients meet the criteria for SIRS as defined by the consensus conference of the American College of Chest Physicians and the Society of Critical Care Medicine.7 It is therefore not surprising that severe critical illness and multiple organ failure are common in burned patients.
Burn intensive care unit organization
Physical plant
Optimally, a BICU should exist within a designated burn center in conjunction with a recognized trauma center, thus providing the capability to treat both thermal and non-thermal injuries. This unit, however, need not be physically located in the same space as that designated for non-burned trauma patients. In fact, the requirements for the care of wounds in burned patients necessitates additional equipment such as shower tables and overhead warmers, and so separate space dedicated to the severely burned is optimal. This space may be located in a separate hospital with established guidelines for transfer.8
The optimal number of beds in the unit may be calculated by the incidence of moderate to severe burns in the referral area, which in the USA is approximately 20 per 100 000 people per year. The Committee on Trauma of the American College of Surgeons and the American Burn Association recommend that 100 or more patients should be admitted to this facility yearly, with an average daily census of three or more patients to maintain sufficient experience and acceptable access to specialized care.8
Personnel
A BICU functions best using a team approach between surgeons/intensivists, nurses, laboratory support staff, respiratory therapists, occupational and physical therapists, mental health professionals, dietitians, and pharmacists (Box 33.1). The unit should have a designated medical director, ideally a burn surgeon, to coordinate and supervise personnel, quality management, and resource utilization. The medical director will usually work with other qualified surgical staff to provide sufficient care for the patients. It is recommended that medical directors and each of their associates be well versed in critical care techniques, and care for at least 50 patients per year to maintain skills.8 In teaching hospitals, two to three residents or other qualified medical providers should be assigned to the 10-bed unit described above. A coverage schedule should be devised to provide 24-hour prompt responses to problems.
Nursing personnel should consist of a nurse manager with at least 2 years of intensive care and acute burn care experience, and 6 months of management responsibilities. The rest of the nursing staff in the BICU should have documented competencies specific to the care of burned patients, including critical care and wound care.8 Owing to the high intensity of burn intensive care, at least five full-time equivalent nursing providers are required per ICU bed to provide sufficient 24-hour care. Additional personnel are required for respiratory care, occupational and physical therapy, and other support. A dedicated respiratory therapist for the burn unit at all times is optimal.
Owing to the nature of critical illness in burned patients, complications may arise that are best treated by specialists not generally in the field of burn care (Box 33.2). For these reasons, these specialists should be available for consultation should the need arise.
Box 33.2 Consultants for the BICU
| •General surgery | Pediatrics |
| •Plastic surgery | Psychiatry |
| •Anesthesiology | Cardiology |
| •Cardiothoracic surgery | Gastroenterology |
| •Neurosurgery | Hematology |
| •Obstetrics/gynecology | Pulmonology |
| •Ophthalmology | Nephrology |
| •Orthopedic surgery | Neurology |
| •Otolaryngology | Pathology |
| •Urology | Infectious disease |
| •Radiology |
Equipment
The equipment needs of the BICU are those items which are common for all ICUs, and some which are specialized (Box 33.3). Each BICU bed must be equipped with monitors to measure heart rate, continuous electrocardiography, non-invasive blood pressure, invasive arterial and venous blood pressures, end-tidal carbon dioxide monitoring, and right heart cardiac output using dilution techniques or data derived from arterial pressure tracings. Continuous arterial blood oxygen saturation measurement is also required, but continuous mixed venous saturation monitoring or the technical equivalent is optional. Equipment to measure weight and body temperature should also be standard. Oxygen availability and at least two vacuum pumps must be present for each bed.
Box 33.3 Equipment for a fully equipped BICU
• Monitors (heart rate, electrocardiography, blood pressure, cardiac output, oxygen saturation, temperature)
• Laboratory support (blood gas analysis, hematology, chemistry, microbiology)
Cardiovascular monitoring
Arterial lines
Measurement of arterial blood pressure is the mainstay for the assessment of tissue perfusion. In critical illness, this measurement can be made using cuff sphygmomanometers; however, in practice this technique is not useful because the measurement is episodic, and placement of these cuffs on burned extremities is problematic. Diastolic pressures can also be artificially elevated in the elderly and obese. Instead, continuous monitoring for hemodynamic instability through the use of intra-arterial catheters is generally preferable while in the ICU for a prolonged period. Lines are typically placed in either the radial or the femoral artery. The radial artery is the preferred site for most critically ill patients because of safety with the dual arterial supply to the hand should a complication occur. However, it has been shown that radial artery catheters are inaccurate in the measurement of central blood pressure when vasopressors are used9 and are notoriously inaccurate in children because of greater vascular reactivity.10 For these reasons, we recommend femoral arterial blood pressure measurement in most burned patients.
Evidence of catheter infection hallmarked by purulence and surrounding erythema should instigate removal of the catheter, which will often suffice. With continued evidence of infection, antibiotics and incision and drainage of the site should be entertained. Great caution must be exercised to avoid arterial bleeding if an incision is made over the catheter site. If a pseudoaneurysm is encountered after arterial catheterization and removal without signs of distal ischemia, compression with a vascular ultrasound device until no further flow is seen in the pseudoaneurysm will often alleviate the problem without operative intervention.11
Cardiac output measurement
The use of pulmonary artery catheters, however, has come under scrutiny from reports indicating no benefit from their use. A study of 5735 critically ill adults in medical and surgical ICUs showed an increase in mortality and use of resources when pulmonary artery catheters were used. Most of these patients had medical conditions. The authors of this report suggested that their results should prompt a critical evaluation of the use of pulmonary artery catheters under all conditions.12 This was followed by a clinical trial in the UK which showed no benefit to the use of pulmonary artery catheters in a general ICU setting.13 These data then suggest that this modality has little utility in today’s practice. In fact, over the past few years, the use of pulmonary artery catheters has significantly diminished except in special circumstances such as unexpected response to treatment such as volume replacement for oliguria. Even in this condition, new technology based on arterial waveform analysis gives an estimate of cardiac output and end-diastolic volume, which generally gives enough information to guide appropriate therapy.14
The base deficit is the stoichiometric equivalent of base required to return the pH to 7.40. Base deficit is routinely calculated on blood gas analysis, and provides a reasonable estimate of the degree of tissue anoxia and shock at the whole body level, particularly in hemorrhagic shock. A rising base deficit indicates increasing metabolic acidosis, and may stratify risk of mortality in patients after major trauma.15 The same can be said for the use of base deficit in burn resuscitation in burned patients.16,17 These studies showed a correlation of higher base deficit and increased mortality, and some have suggested this value is a better monitor of resuscitation than the time-honored monitors of urine output and arterial blood pressure.18 Recent studies in burned patients showed that base deficit was higher in non-survivors during resuscitation, although the authors could not identify a specific boundary for the effect.19,20 Despite its utility as an indicator of shock, base deficit remains a non-specific indicator of metabolic acidosis, and may be elevated with alcohol, cocaine, and methamphetamine use. Interpretation may be difficult under these circumstances.
Lactate is another common measure used to determine adequacy of tissue perfusion. Under acute low-flow conditions, cells transition from primarily aerobic metabolism to anaerobic metabolism for energy production (ATP). A byproduct of anaerobic metabolism is lactic acid. Under ischemic conditions, then, plasma lactate concentration will increase, leading to a decrease in pH. Measurement of lactate is commonly performed to determine the adequacy of generalized perfusion; increases suggest ischemia. Investigators showed that lactate does increase (along with base deficit) in burned patients during resuscitation, and higher levels are associated with poorer outcomes.20 Later in the course, however, lactate concentrations must be used with some caution, as elevated levels do not necessarily indicate ischemia. Under hypermetabolic conditions, which are common in the severely burned, pyruvate dehydrogenase activity is sufficiently inefficient such that lactate levels might be elevated without ischemia. Isolated elevations of lactate should then be interpreted with caution, and confirmation of ischemia or shock by physical or other laboratory findings should be sought.
Transesophageal echocardiography
Transesophageal echocardiography has been used for a number of years as an intraoperative monitor in high-risk cardiovascular patients. It has not been used extensively in other critically ill patients because of the lack of available expertise and paucity of equipment. Since this device can be used as a diagnostic tool for the evaluation of hemodynamic function, it stands to reason that it could be used as a monitor in critically ill severely burned patients. A report documented the use of transesophageal Doppler measurements of cardiac output in a series of severely burned patients showing that intravascular volume and cardiac contractility are significantly diminished the first day after burn in spite of high-volume resuscitation.21 For the monitoring of burn resuscitation, it seems that most use urine output; however, the question has been raised whether this is the proper measure. Investigators in China asked whether esophageal Doppler monitoring of heart function might be an improvement. They studied 21 patients with massive burns (79 ± 8% TBSA burned) who were resuscitated with a goal of 1.0 mL/kg/h. They found that cardiac output was predictably low after injury and increased linearly with time by increases in preload and contractility and decreased afterload. However, changes in cardiac output were most closely associated with increased cardiac contractility and decreased afterload rather than increases in preload. Additionally, urine output was not closely associated with cardiac output.22 These results call into question the validity of urine output as the primary measure of adequacy of resuscitation. Perhaps this is not the best method? A similar study by investigators in Sweden studying the role of cardiac function measured by echocardiography and myocyte damage measured by troponin abundance in the serum showed that half their patients had myocardial damage during resuscitation that was universally associated with some temporary cardiac wall motion abnormality. However, systolic function was not adversely affected.23 We look for further work in the future with regard to the optimal method of assessment of resuscitation; for the present, however, urine output remains the standard.
Mechanical ventilation
Indications for intubation
Intubation entails passing an endotracheal tube from either the nose or the mouth through the pharynx into the trachea. This tube is then connected to a mechanical ventilator to cause inspiration and passive exhalation through the lungs. Indications for intubation in burned patients are in general to improve oxygenation and ventilation, or to maintain gas exchange during clinical conditions expected to compromise the airway (Table 33.1).
Table 33.1 Clinical indications for intubation
| Criteria | Value |
|---|---|
| PaO2 (mmHg) | <60 |
| PaCO2 (mmHg) | >50 (acutely) |
| P/F ratio | <200 |
| Respiratory rate | >40 |
| Respiratory/ventilatory failure | Impending |
| Upper airway edema | Severe |
Ventilatory modes
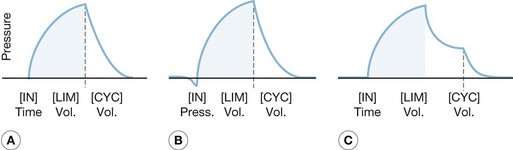
The complexity of mechanical ventilators has increased dramatically since the first generation of volume cycle ventilators used in the 1960s. The development of positive end-expiratory pressure (PEEP) to maintain functional residual capacity was followed by the development of modes using partial ventilatory support, such as intermittent mandatory ventilation in the 1970s. Efficient microprocessors were then developed that permitted modes of ventilation such as pressure support ventilation, time-cycled pressure control ventilation, and inverse ratio ventilation. Most recently, new processors have been used to combine modes of ventilation, such as pressure support and time-cycled pressure control ventilation, and open lung strategies such as airway pressure-release ventilation, with some success (Fig. 33.1). However, even with these new developments, the function of mechanical ventilators remains identical to those first used. Whether these new ventilators have had any appreciable effect on mortality remains to be fully elucidated, though new evidence suggests that choice of mode may make a difference.
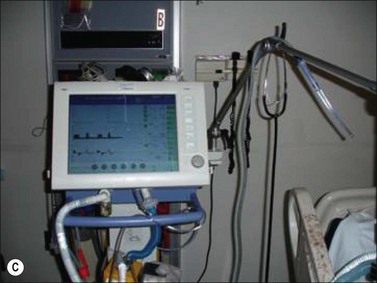
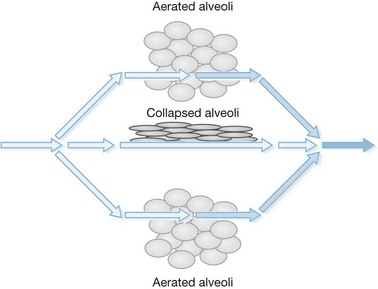
The principal difference in mechanical ventilation from spontaneous ventilation, which each of us use every minute of the day, is the effect of positive pressure as opposed to normal physiologic negative pressure. The use of positive pressure improves gas exchange by recruiting alveoli and increasing functional residual capacity (i.e., the number and volume of open alveoli at the end of expiration), thus improving ventilation perfusion mismatch and reducing intrapulmonary shunting of blood past non-ventilated lung areas (Fig. 33.2). Adverse effects of positive-pressure ventilation lie in its propensity to produce trauma to the airways (barotrauma) and its effects on intrathoracic pressure, which can impede venous return to the heart and thus reduce cardiac output.
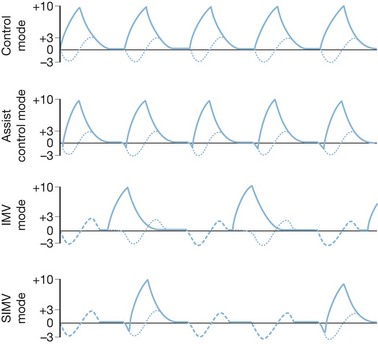
Control and assist control ventilation
The volume control modes are volume-cycled settings that deliver a preset tidal volume at a minimum respiratory rate and inspiratory flow rate regardless of the patient’s own respiratory efforts (Fig. 33.3). Of the two, the control mode will not trigger with patient effort. This mode is typically very uncomfortable for patients owing to ventilator–patient dyssynchrony, with use limited to patients under general anesthesia or heavy sedation. The assist control mode differs in that a breath will be delivered regularly according to a prescribed rate, but will also deliver a breath upon patient negative-pressure effort to open a flow valve, at which time the ventilator will fire, allowing the patient to control his or her own ventilatory rate with a preset minimum rate as a back-up. This mode is typically used in heavily sedated patients who cannot generate enough tidal volume under pressure support modes or intermittent mandatory ventilation mode, or in those patients in whom the clinician wishes to minimize the work of breathing. It must be noted, however, that the work of breathing can be increased dramatically in patients with excessive ventilator triggering; increasing the minimum number of breaths to match the patient’s effort can minimize this effect. When using this mode of ventilation, it is important to monitor lung compliance by measuring serial peak and plateau pressures for diagnostic purposes as well as minimization of barotrauma.
Intermittent mandatory ventilation
Intermittent mandatory ventilation (IMV) was developed to allow spontaneous ventilation interspersed with volume-cycled or time-cycled pressure control mechanical ventilation. It was developed initially as a method to wean patients from the ventilator by depending more and more on patient effort for ventilation. The addition of a synchronized mode (SIMV) to avoid placing a mechanical breath on top of a spontaneous patient breath greatly improved this mode. It has the advantage of maintaining some patient work in breathing to preserve respiratory strength when mechanical ventilation is required, and as a weaning tool to progressively increase patient effort while reducing mechanical support in preparation for discontinuing mechanical ventilation. This method can be problematic in patients with low pulmonary compliance, and the IMV mode may not allow for sufficient spontaneous tidal volumes due to extremely limited inspiratory capacity. The addition of pressure support to augment spontaneous ventilatory efforts can be used (Fig. 33.4).
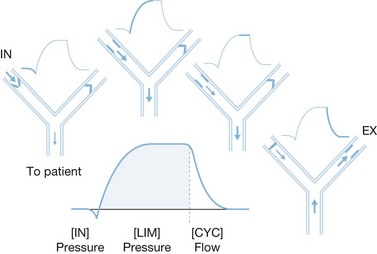
Pressure support ventilation
Pressure support ventilation is a patient-triggered pressure-limited flow-cycled ventilatory mode (Fig. 33.5). Each pressure support breath is triggered by patient negative-pressure effort, at which time a valve opens producing a high-flow pressure-limited breath. The breath ends when the patient’s inspiratory demand falls below a preset limit, allowing spontaneous respiration and complete patient control. It also differs from the other modes in that it is flow-cycled as opposed to volume- or pressure-cycled. Because this mode is triggered and completed entirely by patient response, it cannot be used in patients with decreased respiratory drive, such as paralyzed patients and those who are heavily sedated. This mode has a number of advantages because of improved synchrony with patient effort. It can be used to provide full ventilatory support by setting a flow and pressure such that an adequate tidal volume is delivered. It can also be used effectively during weaning by reducing the pressure incrementally to allow for progressively greater patient effort. Caution must be exercised when reducing ventilatory support so that a sufficient tidal volume is still delivered to maintain adequate ventilation.
Inverse ratio ventilation
The beneficial effects of inverse ratio ventilation have been questioned. It may be that the same effect can be gained simply by increasing peak airway pressures with PEEP or peak inspiratory pressures. In fact, some studies have showed no benefit of inverse ratio ventilation compared to conventional volume ventilation in terms of oxygenation.24 These studies did show some slight improvements in ventilation (Paco2). For this reason, inverse ratio ventilation cannot be recommended except in the setting of ARDS refractory to other therapies.
High-frequency oscillatory ventilation
High-frequency oscillatory ventilation (HFOV) is a mode of ventilation that delivers subtidal, high-frequency oscillatory breaths at a constant mean airway pressure (mPAW), otherwise described as ‘CPAP with a wiggle.’ It is thought to maintain open alveoli by recruiting collapsed portions of diseased lung and applying the equivalent of continuous positive airway pressure used during conventional ventilation. Optimizing mean airway pressure limits peak pressure, thereby making this a ‘lung-protective’ mode. A recent randomized prospective trial in adult patients with ARDS demonstrated that HFOV resulted in brief improvements in oxygenation although mortality and complication rates were not different.25 In burned patients, one center reported early success in reversing profound hypoxemia in patients with ARDS while facilitating early excision and grafting with intraoperative use.26 However, this same group recently showed that in burned patients with inhalation injury this method often failed secondary to hypercapnia rather than hypoxia.27 It stands to reason, therefore, that this method might be tried in those with oxygenation difficulties, and perhaps continued if hypercapnia is less of an issue.
High-frequency percussive ventilation
High-frequency percussive ventilation (HFPV) is a pressure-limited, time-cycled mode of ventilation that delivers subtidal pressure-limited breaths at a high frequency (400–800 beats/min) superimposed on a conventional inspiratory and expiratory pressure-controlled cycle (10–30 breaths/min). The purported advantages are to mobilize airway secretion and casts for better pulmonary toilet, and to provide adequate gas exchange at lower airway pressures. This method of ventilation has been tested primarily in burned patients with inhalation injury, and was first reported in 1989.28 In this study, HFPV was used as a salvage therapy in patients with inhalation injury and as primary therapy in another group. Improvements in oxygenation and a lower rate of pneumonia were seen. Another study documented an improvement in mortality in burned patients with inhalation injury treated with HFPV compared to historical controls.29 Other outcomes in this study were significant decreases in the work of breathing, and lower inspiratory pressures in addition to improvements in oxygenation and the rate of pneumonia. This method of ventilation seems to be particularly efficacious in the treatment of inhalation injury, particularly in comparison to HFOV.
A recent study was performed in severely burned ventilated patients testing the effects of low tidal volume conventional ventilator strategy against an HFPV ventilator strategy. This study again found differences in improved oxygenation early in the course, but no differences in mortality or other outcome measures. However, the study did show that the need for ventilator ‘rescue,’ defined as the need for changing ventilator modes due to inadequate oxygenation or ventilation despite maximizing the mode, was higher in the ARDSnet group, thus favoring the use of HFPV in this population (i.e. less rescue indicated). At the very least, these data show that HFPV is useful in the population of burned patients, and may in fact be beneficial by reducing the risk of pulmonary failure at least in the short term.30