(1)
Department of Plastic, Reconstructive and Cosmetic Surgery, University Hospitals Leuven, Leuven, Belgium
Abstract
In the following study we report on the development and application of the cremaster chamber model. Seventeen male Sprague-Dawley rats were studied in two experimental groups. In the control group, Group I, the muscle flap was preserved in the medial border of the rat’s hind limb. For evaluation the cremaster was removed from the leg and prepared for in vivo observations after 24 h. In Group II, the cremaster muscle chamber was implanted and in vivo observations were performed in post-operative intervals of half an hour, 24, 48 and 72 h, respectively. The study proved that the cremaster chamber model was equal to the classic cremaster muscle preparation for microcirculatory measurements for at least 24 h.
Keywords
CremasterMusclePlexiglasObservation chamber and microcirculationIntroduction
Sandison [1] in 1924 was the first to implant an observation chamber into an animal to study the microcirculation of living tissues. Since then, various chambers have been developed and implanted with the aim to investigate the microcirculation in small animals [1–14], and even in human subjects [15]. Transparent chamber techniques have proved useful for the long-term observation of the microvascular system and surrounding tissues under many experimental conditions. The tissue under microscopic observation was either preformed tissue (mostly skin, striated muscle and subcutaneous tissue) [16–18] or newly formed granulation tissue [2, 8, 12, 19]. The rat cremaster muscle is widely used in acute microcirculatory studies because of its transparency and convenience of harvest. Based on this successful and well-established model we applied a similar technique to a cremaster muscle flap to develop a method for long-term in vivo studies [20–22]. This model, however, has several disadvantages; firstly, multiple animals are needed to study long-term events within the same experimental set-up. Furthermore, an additional operation is needed after the initial preparation of the cremaster muscle.
In the study presented in this chapter our aim was to develop a model that would be equal to the existing cremaster preparation but would allow for permanent chronic microcirculatory measurements within the same muscle over time. We report the assembly, implantation and the first in vivo microcirculatory observations of a muscle island flap in a transparent chamber that allowed for 3 days of consecutive measurements.
Materials and Methods
All experimental procedures and protocols described were approved by the institution’s animal care and use committee and followed National Institutes of Health guidelines for the humane treatment of animals. Sprague-Dawley (SD) rats weighing 130–180 g were used. The rats were individually caged at room temperature on a 12-h light/dark cycle with free access to laboratory chow and water. For all procedures the rats were anesthetized by peritoneal injections of pentobarbital (60–70 mg/kg) and given supplements (20 % of the initial dose) as needed. Body temperature was maintained at 35–37 °C with a servo-controlled heat lamp throughout each procedure.
After the muscle tube was isolated (described later), the 17 SD-rats were assigned to two main experimental groups: the control group (N = 8), which received no chamber, and the chamber group (N = 9), which received the chamber. In the controls, the isolated cremaster tube flap was left intact and inserted into a subcutaneous tunnel on the medial border of the hind limb. In the chamber group the chamber was implanted after muscle isolation. Evaluations were done at 30 min post-implantation and at 24-h intervals in both groups for the first three post-operative days in the chamber group.
Cremaster Muscle Plexiglas Chamber
The chamber consisted of three parts (Fig. 15.1). The bottom portion of the chamber is a Plexiglas frame (measuring 38.1 mm in diameter). It has six suture-slides placed at 45° angles, which permit the cremaster muscle to be stretched over the top of this part of the chamber. The top frame or observation part of the chamber is identical to the bottom, with the difference being that a circular window (23 mm in diameter) is placed centrally. The last part of the chamber is a cover glass that fits exactly in this window. For implantation, the cremaster muscle is sandwiched between the top and the bottom frames. Using six stainless steel micro-screws as spacers, the frames are secured to each other in such a manner to avoid compressing any vessels. Fixation of the chamber to the skin of the rat is possible, because of the specially designed chamber base. The base is at a 125-degree angle to the frame halves and has six suture holes in both the top and the bottom of the base to fixate the skin.
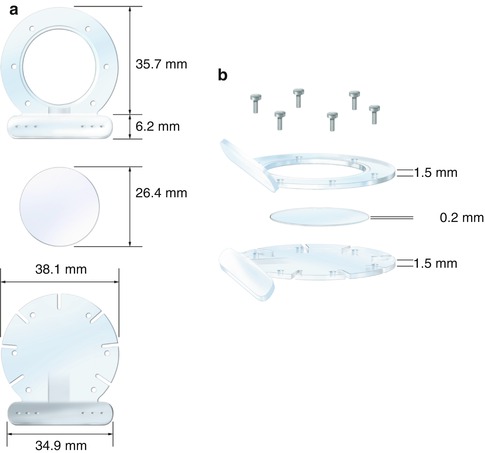

Fig. 15.1
The cremaster muscle chamber. (a) Front view of the chamber. (b) Side view of the chamber
Cremaster Muscle Isolation and Chamber Implantation
The cremaster muscle on its neurovascular pedicle was dissected as described previously [20, 21]. The right inguinal area was shaved and swabbed with povidone-iodine (betadine). With the aid of an operating microscope (Zeiss Opmi 6, Goettingen, Germany), the external iliac artery was dissected and freed up to the origin of the epigastric pedicle. The entire blood supply to the flap was introduced via the pudic-epigastric pedicle by ligating or coagulating all collateral vessels [23]. The cremaster muscle flap was spread on the bottom frame of the Plexiglas chamber with 5-0 silk stay sutures. The muscle was bathed in physiological saline and covered with the round cover glass and the top frame of the chamber to keep it moist and free from dehydration and atmospheric oxygen throughout the experiment. Direct visualization of the cremaster muscle microcirculation under the microscope allowed for compression free fixation of the screws. The base of the chamber was fixed to the body of the rat along the inguinal canal (Fig. 15.2). The muscle was allowed to recover for 30 min between implantation of the cremaster muscle chamber and the initial microscopic investigation was allowed to eliminate the effects of immediate surgical trauma on the chamber tissue. Microcirculatory measurements were performed immediately and at 24-h intervals for 3 days following chamber application.
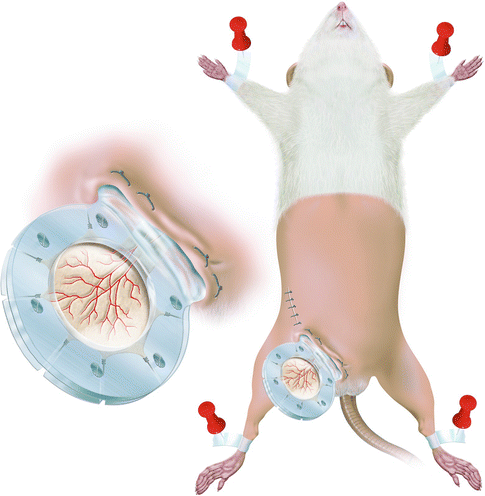

Fig. 15.2
The cremaster muscle chamber is fixed to the rat along the inguinal canal after shaving the lower half of the rat. (Inset) Close-up of the chamber with the consecutive branch orders of the cremaster muscle vessels
Muscle Isolation and Preparation in the Control Group
In the control animals, the cremaster muscle was isolated as in the group receiving the chamber. Instead of implanting the chamber, the tube flap was left intact and inserted into a subcutaneous tunnel on the medial border of the rat’s hind limb as described previously [22]. The muscle tube was anchored with a pull-out suture at the ankle level. For measurements the tube flap was withdrawn from the leg and prepared for microcirculatory observation. The rat was secured on a tissue bath and the cremaster muscle was spread over a cover glass and fixed with 5-0 silk stay sutures. The muscle was kept moist and covered with impermeable film (Saran Wrap presoaked in distilled water).
Intravital Microscopy
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








