Summary and Key Features
- •
Metal ions have been used in topically applied skin care treatments for centuries.
- •
While their use was initially based on empiricism, scientific data now explains many of the observed effects.
- •
Well-established benefits for zinc include healing damaged skin as well as damage prevention.
- •
Copper has also been established to support skin damage repair processes.
- •
Selenium benefits appear to be tied to its antioxidant activity.
- •
Other metals such as aluminum and strontium are used in more narrow applications.
Introduction
Are certain topically applied metal ions simply innocuous treatments or do they provide real technical benefit? Their use goes back to the earliest recorded medical text (ca. 1500 BC), the Ebers papyrus of ancient Egypt. For example, calamine (a natural material containing zinc oxide) was described for treating many skin and eye ailments; green copper-based minerals (likely malachite) were used for burn wounds and itching. Many of these applications have withstood the test of the ensuing 3500 years of history, providing a first clue of real technical merit; as an example, zinc is still the first choice to soothe a crying baby’s bottom.
This anecdotal support for the importance of metal ions is substantiated by more rigorous investigations, such as those that describe the impact of nutritional deficiencies. A deficiency of zinc can occur either by diet or as a result of a genetic condition that blocks the intestinal uptake, resulting in acrodermatitis enteropathica (AE). AE manifests itself as severe dermatitis in the vicinity of the mouth, nose, ears, and anal areas (orifices), and on the skin and nails of the fingers and toes (acra). Likewise, a disease resulting in copper deficiency, Menkes syndrome, causes defective keratinization in skin and hair growth, manifested by the formation of kinky hair.
While ancient empiricism, practical utility, and clinical manifestations of deficiency support the conclusion that metal ions are important to skin health, a deeper level of understanding is required to confirm this. A molecular basis for these empirical and clinical observations is beginning to emerge that provides strong reinforcement of the links between metal ions and skin condition.
This chapter will focus specifically on five metals—zinc, copper, selenium, aluminum, and strontium—which are currently used in cosmeceuticals. Each metal will be covered sequentially, reviewing commonly used materials followed by clinical and scientific data supporting their use. There are many other metals that have found usage in cosmeceuticals (see Table 10.1 ). Searching the skin-related literature (Medline 1993–present) demonstrates that there is substantial scientific activity exploring the technical basis for utilization of some of these metals. Also summarized in Table 10.1 for some of the metals is the introduction of new personal care products containing them; substantial commercial activity is evident.
| Metal | Potential Benefits | Medline Hits a | New Products b |
|---|---|---|---|
| Zinc | Cellular growth, wound healing, photoprotection, antioxidant | 675 | 10,339 |
| Copper | Keratinization, collagen formation, hair growth, energetics | 374 | 2031 |
| Iron | Oxygenation, microcirculation | 598 | NA |
| Selenium | Antioxidant, antifungal | 201 | 647 |
| Aluminum | Antiperspirant | 349 | NA |
| Strontium | Antiirritant | 27 | 51 |
| Silicon | Connective tissue formation | 200 | NA |
| Magnesium | Maintenance of cutaneous health | 179 | NA |
| Calcium | Cellular adhesion, antiinflammatory, epidermal maturation | 2274 | NA |
| Chromium | Microcirculation | 161 | NA |
| Silver | Antibacterial | 624 | NA |
| Titanium | Photoprotection | 245 | NA |
a Skin-related citations (1993–present).
b New personal care products assessed from ingredient statements within Mintel Group Global New Products Database. For metals that have many nonbiologic benefit uses (e.g., colorants, thickeners), data are excluded (NA) due to the inability to separate those from the group.
Zinc in Cosmeceutical Products
Materials
There are 55 different zinc-containing materials listed in the International Cosmetic Ingredient (INCI) Dictionary and Handbook (a tabulation of all materials used in cosmetic and personal care products). Of those, seven have been approved by the US Food and Drug Administration (FDA) for over-the-counter (OTC) usage as safe and effective for a range of benefits, including skin protection, antimicrobial activity, and astringency ( Table 10.2 ). The skin protective benefits of these zinc materials find applications treating various inflammatory dermatitis conditions such as poison ivy and diaper rash. The wide range of zinc materials approved by the FDA provides a strong indication of the general utility of zinc as an effective treatment.
In most of these materials, zinc ion itself appears to be the primary source of the benefit. All of these materials utilize zinc in its ionic form (Zn 2+ ) with different counterions that result in an electrically neutral compound. These counterions can modulate the solubility and bioavailability of the zinc species itself. For example, zinc sulfate is water soluble whereas zinc oxide is only sparingly soluble. Zinc sulfate would be expected to be highly available initially with rapid depletion whereas zinc oxide tends to have a lower level of initial activity but sustained for a long time. By choice of the specific material, the cosmeceutical formulator can tailor the physical properties and activity to the product function.
The other zinc-containing materials utilized in cosmeceuticals but not specifically accepted by the FDA for OTC drugs can likewise be expected to have the potential to deliver zinc-based benefits. However, since the use of these materials is not as widespread, the product formulator must exhibit greater pharmacology expertise to ensure that the intended benefits are delivered; bioavailability becomes a complex interaction of material interacting with the product matrix.
Basis for Use of Zinc Materials
Clinical Perspective
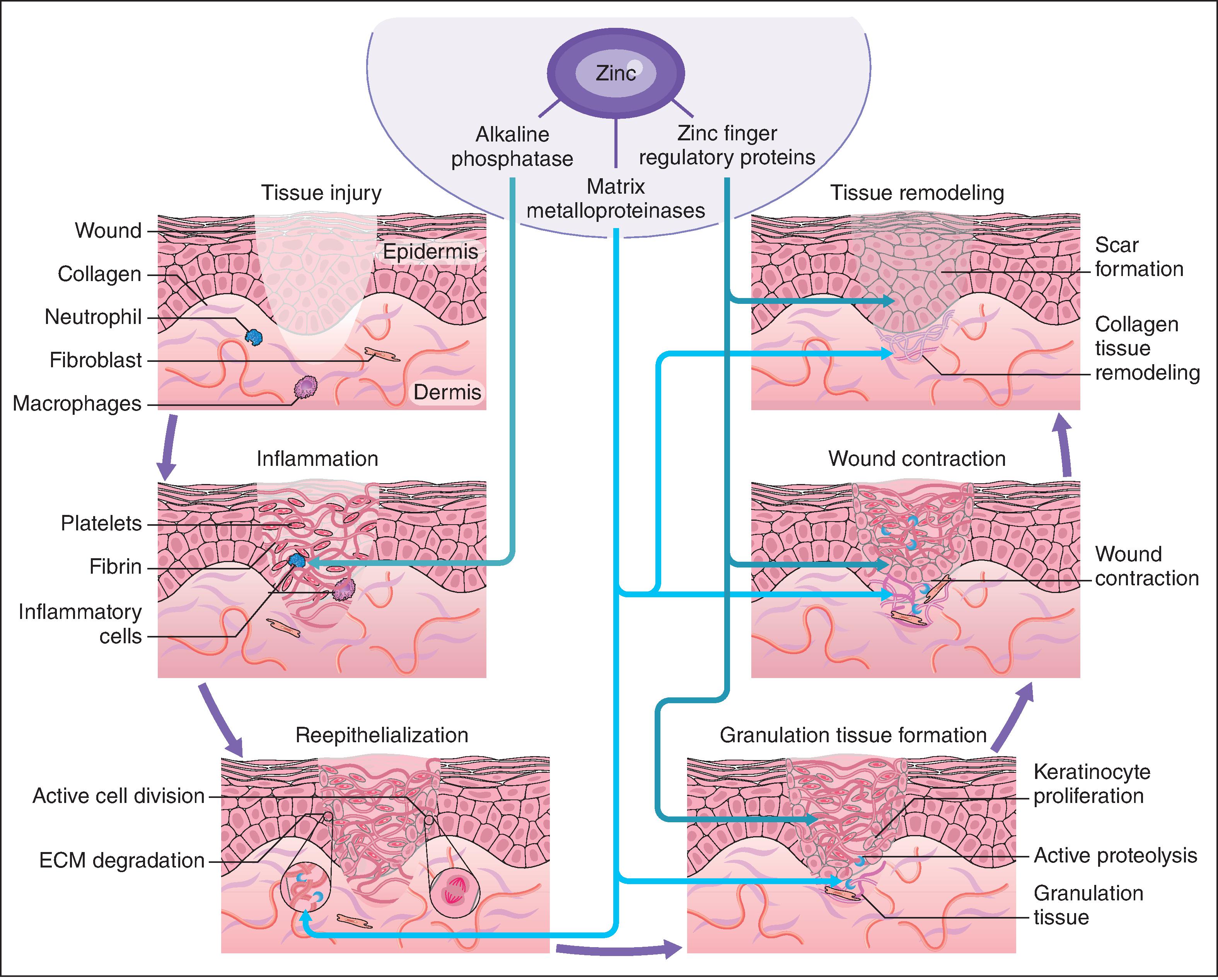
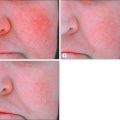
Damaged skin repairs itself in a very complex process. In the case where the damage is physical and a wound results, a well-defined process ensues: inflammation, reepithelialization, granulation tissue formation, wound contraction, and tissue remodeling. During wound healing, the requirement for zinc increases dramatically. In rat wound models, local zinc levels are seen to increase after wounding, demonstrating the physiologic need for this metal in the repair process. Topically applied zinc compounds have been shown to speed repair, for example, in leg ulcers; the rate of delivery of zinc to the damaged site may be initially rate limiting in the repair process. The rate of reepithelialization was increased with topical zinc in a pig model; the nature (bioavailability) of the zinc material was found to be important—sparingly soluble zinc oxide was superior to soluble zinc forms. An indirect measure of local zinc ion activity at a wound repair site comes from monitoring metallothionein (MT), which is responsible for the storage and delivery of zinc to other proteins and enzymes requiring zinc for their function. MT upregulation can be found in vivo by exposure to zinc; treatment of keratinocytes in vitro with a material that selectively binds zinc inhibits the upregulation of MT and slows cellular proliferation.
Where the damage to skin is more chemical in nature, the dominant manifestation is inflammation. There is a growing body of evidence that zinc has antiinflammatory activity. Zinc reduces the irritancy caused by surfactants in the oral cavity. This effect has been observed in vitro as well as in skin cultures by monitoring interleukin (IL)-1α production and demonstrating that pyrithione zinc inhibits surfactant-induced IL-1α release. The inflammatory conditions bullous pemphigoid and decubitus ulcers are accompanied (caused?) by low serum zinc levels. The antiinflammatory benefits of zinc most likely also play a role in the wound-healing process discussed earlier. In addition to facilitating repair processes, zinc appears to confer a protective function via providing antioxidant activity. Zinc has been shown to reduce the cellular and genetic damage caused by exposure to ultraviolet (UV) light and enhance resistance of skin fibroblasts to oxidative stress.
Zinc has also recently been shown to improve skin elasticity, reducing the signs of aging skin, and has recently been implicated in hair loss.
Scientific Foundation
An average human contains 2.5 g of zinc and requires 15 mg/day to remain healthy (this is exceeded only by iron for trace elements). The vast majority of the zinc is present in metalloenzymes and proteins. This field was opened in 1940 with the discovery that carbonic anhydrase, a ubiquitous enzyme required for maintaining physiologic pH, contains zinc and that the zinc is required for catalytic activity. Since that time, over 300 enzymes requiring zinc for activity have been structurally characterized. Even more impressive are the thousands of zinc-containing proteins that require zinc for conferring a three-dimensional structure that allows them to regulate replication of DNA and transcription of RNA. These proteins form the class called zinc fingers and regulate the fundamental biologic process of transcribing genetic information to functional proteins. At least 3% of all proteins encoded for by the human genome have zinc fingers; this has led Berg to coin the phrase “galvanization of biology” to acknowledge the importance of this metal in human physiology.
While it is beyond the scope of this chapter to review many of the zinc-containing biomolecules (see Table 10.3 for an overview of important examples), a few will be highlighted that have specific relevance to cutaneous biology and support the clinical observations reviewed earlier. Matrix metalloproteinases (MMPs) are zinc-dependent proteases capable of degrading many molecules important in wound healing, including signaling factors as well as the structural proteins of the extracellular matrix (including collagen and elastin). Wound healing requires intensive cell division and protein synthesis, thus the zinc finger proteins DNA and RNA polymerases are critical throughout this process. The antiinflammatory role observed clinically for zinc, relevant both in wound healing as well as in other dermatitis conditions, may lie partially in the importance of alkaline phosphatase (AP). AP requires multiple zinc ions and is involved in adenosine monophosphate metabolism, which has a role in restraining an inflammatory response. Recent research also highlights the ability of zinc to modulate the keratinocyte surface expression of Toll-like receptor 2 (TLR2), which would inhibit the production of some inflammatory cytokines. The breadth of the impacts of these zinc biomolecules on the wound-healing process is represented schematically in Fig. 10.1 .
| Enzyme | Chemical Function of Zinc | Physiologic Function of Biomolecule | Relevant to Skin? |
|---|---|---|---|
| Alcohol dehydrogenase | Catalyze oxidation of alcohols (esp. ethanol) to aldehydes | Liver metabolism | |
| Carboxypeptidase | Catalyze hydrolysis of C-terminal peptide residues Protein digestion for nutrition | ||
| Thermolysin | Catalyze hydrolysis of peptides | ||
| Matrix metalloproteinases Collagenase (MMP-1) Elastase (MMP-12) Gelatinase (MMP-2) | Catalyze hydrolysis of matrix proteins | Formation of extracellular matrix Hydrolysis of collagen Hydrolysis of elastin Hydrolysis of gelatin | X |
| β-Lactamase | Catalyze hydrolysis of β-lactam rings (e.g., penicillin) | ||
| Carbonic anhydrase | Catalyze hydration of CO 2 | Physiology of CO 2 transport and physiologic buffering | |
| Nuclease P1 | Catalyze formation of 5′ single-stranded nucleotides from RNA and DNA | ||
| Superoxide dismutase | Catalyze dismutation of superoxide anion into O 2 and H 2 O 2 | Scavenges damaging superoxide | X |
| Phosphotriesterases | |||
| Alkaline phosphatase | Catalyze hydrolysis of phosphate monoesters | X | |
| Leucine aminopeptidase | Catalyze hydrolysis of leucine N-terminal peptide residues | ||
| Phospholipase C | Cleaves bond between head group and lipid moiety of phospholipids | ||
| Metallothionein | Binding of zinc | Storage of zinc | X |
| Zinc finger class DNA polymerases RNA polymerase | Confers conformation to facilitate nucleotide binding | Nucleic acid metabolism Replication of DNA Transcription of RNA | X |
| α-Amylase | |||
| Aspartate transcarbamylase |