Controlled Tissue Expansion in Facial Reconstruction
History
Tissue expansion can be a natural physiologic process encountered in normal life, pregnancy and obesity being the most obvious examples. This process has been used for centuries in certain cultures to modify facial features as illustrated on the pages of National Geographic magazine. Modern use of tissue expansion was first introduced in the early 1900s. A technique was described that used progressively larger objects to expand tissue with multiple operations required.1 Because of the number of procedures required and the high morbidity, the procedure was abandoned. The practical application of tissue expansion was not possible until the introduction of plastic materials later in the century. In 1957, Neumann published “The Expansion of an Area of Skin by Progressive Distension of a Subcutaneous Balloon.” This article described the use of a latex balloon to expand the skin for reconstruction of an ear.2 He placed the balloon above the ear in a subcutaneous tissue pocket and brought a polyethylene tube out through an independent skin incision. He gradually injected air into the implant through a stopcock. His procedure was a success, but the potential impact of his work was not recognized at the time. No other work was performed on tissue expansion for almost 20 years.
In the early 1970s, Radovan and Austed independently began studying the use of tissue expansion to repair soft tissue defects. Radovan’s early expanders consisted of a balloon and two separate tubing systems for injection and removal of saline. The entire system was placed under the skin.3 His first experiments were performed in 1976 and were met with skepticism when his results were presented to the medical community later that year. This was most likely because of his unscientific methodology. He was not discouraged by the skepticism and continued his clinical trials. The results of Radovan’s experiments met wider acceptance 3 years later accompanied by Austed’s work using an osmotically driven, self-inflating expander.4 Austed, unaware of Neumann’s and Radovan’s work, first suggested the idea of implanting a device beneath the skin to induce tissue expansion in 1975. His work established the basis for understanding of the tissue response to expansion. His early laboratory experience and histologic studies confirmed that tissue expansion is safe and effective.5
Physical Properties and Morphologic Changes
It is important for surgeons performing tissue expansion to understand the physiologic and morphologic changes associated with the process. This knowledge will help the surgeon understand the potential benefits, limitations, and complications of this procedure. When the technique of tissue expansion was first introduced, the unanswered question was whether skin is stretched or new skin is created. If skin is simply stretched, a permanent gain in terms of surface area would not occur. Gibson6 studied the properties of skin and described its inherent extensibility. He noted that skin could stretch beyond its inherent extensibility and called this mechanical creep. The beneficial effects of mechanical creep are attributed to displacement of interstitial fluids and mucopolysaccharide ground substance, parallel alignment of randomly oriented collagen fibers, and migration of tissue into the field by the stretching force.
He referred to biologic creep as the gradual stretching of skin overlying a slowly expanding subcutaneous structure. This is not simple stretching because the skin is affected by metabolic activity, including the creation of epithelium, blood vessels, nerves, lymphatics, collagen, and elastin fibers. All of this occurs during sustained prolonged expansion of the skin.
The first experimental studies of skin expansion were performed by Austed et al5 and Pasyk et al7 using guinea pigs. They used self-inflating expanders, causing gradual uninterrupted expansion. Unlike humans, guinea pigs have loose skin, allowing it to move freely over the muscle beneath and to be picked up in large folds. These qualities make the guinea pig model less than ideal for understanding the effects of expansion on human skin. However, even with these limitations, their studies provided valuable information about tissue expansion. Because pig skin lacks a well-developed panniculus carnosus and has elastic tissue comparable with that of humans, it has been used in place of guinea pig skin for subsequent laboratory studies of skin expansion.
Epidermis
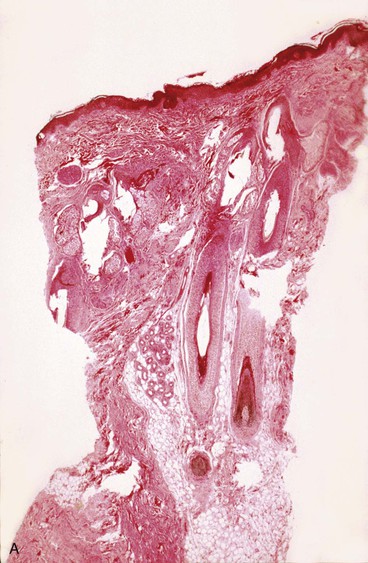
The epidermis is composed of stratified squamous epithelium of the keratinized type. The epidermis shows no thinning during or after expansion. In fact, most studies have noted an increase in thickness during skin expansion. The active basal layer of the epidermis increases in density and thickness. The basal layer normally composes 10% of the thickness of the epidermis but during expansion increases to 40%. van Rappard et al8 described the histologic changes found in expanded pig skin. They observed that during skin expansion, epidermal thickness increases; however, there is no histologic change in the layers of the epidermis. Other studies have also demonstrated that epidermal thickness increases or remains unchanged during expansion in spite of an obvious increase in surface area (Fig. 25-1). These findings would indicate that the epidermis is not merely being stretched. Using tritiated thymidine labeling, Austed et al9 demonstrated an increase in the cellular mitotic rate in the stratum basale of expanded skin and showed a net gain in donor tissue. van Rappard et al10 confirmed these findings in the pig model. They demonstrated that the number of epidermal cells for a given surface area remained the same in expanded and nonexpanded skin. Initial stretching of the epidermis from inflation of an expander increases the intercellular spaces, which in turn probably signals the cells to increase mitotic activity and restore the epidermis to a normal cellular density and thickness. The increase in mitotic activity caused by tissue expansion eventually returns to normal but remains active for as long as 2 months after expansion is discontinued.
Dermis
The dermis, which is a sheet of connective tissue underlying and supporting the epidermis, is composed of the papillary layer and the reticular layer. The papillary layer contains finer collagen fibers than the reticular layer and contains numerous elastic fibers. The reticular layer is composed of dense, irregular collagen fibers. The reticular layer is responsible for the lines of tension. The dermis does not tolerate expansion as well as the epidermis. During tissue expansion, a rapid decrease in dermal thickness is observed and averages 20%.11 Total restoration of dermal thickness after skin expansion has not been demonstrated. The amount of thinning depends on the rate of expansion. Greater thinning is noted with more rapid expansion. The expanded dermis contains large bundles of collagen fibers and active fibroblasts. An increase in collagen synthesis occurs in the dermis during tissue expansion.5 Normal collagen fibers contain bundles cross-linked at angles of 70°. With expansion, these angles gradually decrease until the collagen bundles are almost parallel to the skin surface. This effect is noted for 6 months after cessation of the expansion process.8 Melanin production increases during skin expansion and can cause a temporary hyperpigmentation of the skin. This disappears, in most cases, within a few months. Olenius et al12 described an increase in the amount of melanin pigment granules in the basal layers of expanded human skin.
Subcutaneous Tissue
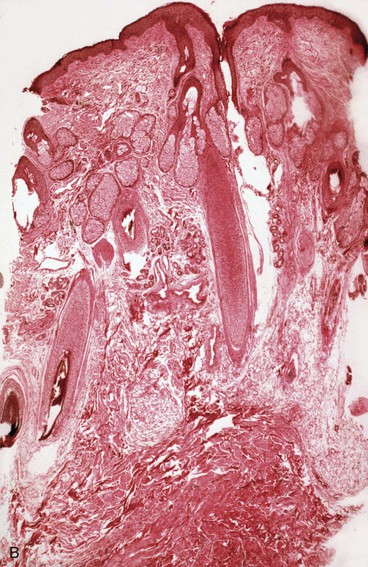
The subcutaneous tissue is composed of fat, loose connective tissue, collagen, arteries, veins, nerves, lymphatics, hair follicles, and sebaceous glands. The thickness of the subcutaneous layer is diminished as much as 50% during prolonged expansion. However, Pasyk et al11 noted the return to greater than normal thickness of the subcutaneous tissue within 2 years after removal of expanders in a series of three patients. During skin expansion, there is a marked decrease in the number of fat cells within the subcutaneous tissue, and those that remain have less lipid content (Fig. 25-2).11 There is also an increase in the interlobular spaces and an increase in the collagen content within these spaces. A varying amount of fat necrosis is also noted. The more rapid the skin expansion, the greater is the fat necrosis. Restoration of normal-appearing fat cells after skin expansion is not observed for as long as 12 months after expansion.8
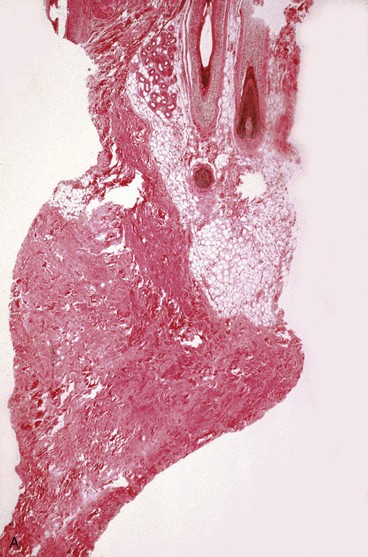
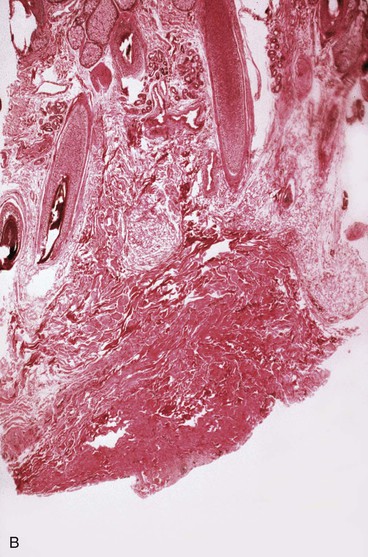
FIGURE 25-2 A, Biopsy specimen of nonexpanded skin. Note presence of numerous fat cells. B, Biopsy specimen of expanded skin in same area. Note absence of fat cells.
Skeletal Muscle
Skeletal muscle is sensitive to tissue expansion. Ultrastructural changes of expanded muscle occur. An increase in the number and size of mitochondria, number of vesicles, and amount of sarcoplasm is observed. The number of muscle cells remains unchanged, but significant thinning of muscle occurs during tissue expansion.8 The striated pattern of muscle cells decreases, and some cells may become necrotic. Hyalinization of muscle cells is also observed and may be followed by calcification in some cases. The significance of these changes is not fully understood, but expansion may lead to a decrease in muscle function and strength in the region of tissue expansion. Sasaki13 has reported visible muscle atrophy and weakness after tissue expansion, but permanent sequelae are rare.
Blood Vessels
Blood vessels undergo some of the most important changes during tissue expansion. Tissue expansion stimulates the proliferation of blood vessels, creating highly vascular tissue (Fig. 25-3). Distention of capillaries is first noted during tissue expansion, followed by an increase in the number of venules and arterioles within a few days. Barium angiography demonstrates a dense vascular pattern in expanded tissue.14 Stark et al15 demonstrated a rapid elongation of arteries and veins with no loss of vessel wall diameter or intimal integrity. They also found no difference in tolerance to tissue expansion between arteries and veins. Studies of flap survival, which is dependent on skin vascularity, reveal that flaps raised from expanded tissue have survival rates comparable to those of delayed flaps. Cherry et al14 demonstrated that flaps raised from expanded tissue have a 117% increase in survival length compared with random pattern flaps raised in nonexpanded skin. Other authors have described similar findings.16,17 In essence, expanded skin has characteristics similar to delayed flaps. These studies indicate that tissue expansion enhances the vascularity of skin and improves flap survival.
Nerves
Nerve tissue appears to tolerate expansion well without demyelination or necrosis of nerve tissue. There are no clinical reports of nerve deficits attributed to tissue expansion. The potential benefit of nerve expansion for nerve grafting or transposition is promising. Elongation of peripheral nerves by use of an expander has been successful. Manders et al18 described their experience with expansion of peripheral nerves and made some important observations. They monitored the intraluminal pressure of expanders while measuring nerve response to expansion with electroneuromyography. They observed no neurologic changes in response to expansion if the intraluminal pressure of expanders remained below 40 mm Hg. If higher pressures were used, reduction in axon potential was immediately noted. They recommend monitoring of intraluminal pressure of all expanders used in patients undergoing expansion of peripheral nerves.
Capsule
A dense fibrous capsule develops around tissue expanders (Fig. 25-4). The capsule is composed of elongated fibroblasts that are active in collagen production.5 Granulation tissue consisting of macrophages, fibroblasts, and lymphocytes is first observed, followed by development of a double-layered capsule within 7 days after an expander is implanted. The inner layer consists of macrophages and the outer layer eventually becomes rich in collagen fibers. Histologic remnants of the capsule can be found up to 1 year after removal of an expander, but grossly the capsule disappears within a few weeks after removal of an expander. The contribution the capsule makes to the blood supply of expanded skin is not fully understood, but in flaps in which capsulectomies have been performed, there appears to be no difference in flap survival compared with those with intact capsules.19 Myofibroblasts are also present in the capsule, and some believe that leaving the capsule intact may add to stretchback. The significance and function of the myofibroblasts are not fully understood.
Tissue Expansion Devices
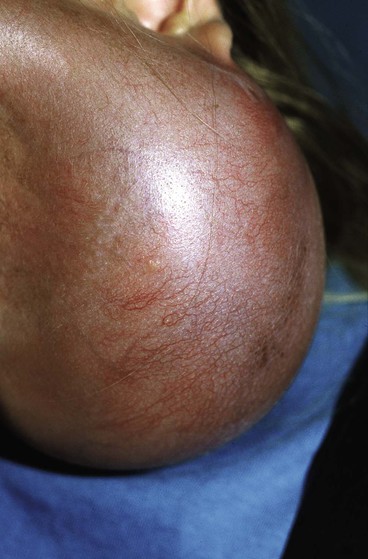
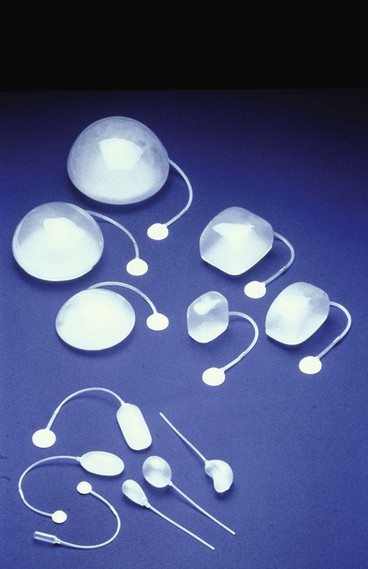
Because nearly every part of the body has been considered for tissue expansion, a wide variety of expander styles, sizes, and shapes have been introduced (Fig. 25-5). Custom-made expanders can also be used for irregular defects or unusual areas. The standard Radovan expander is composed of a Silastic reservoir, a self-sealing injection port, and connecting tubing. This design remains the most commonly used system. Expanders with self-contained injection ports are useful in some situations, but the injection port may be more difficult to identify, leading to a greater risk of damage to the expander during inflation. The necessary multiple percutaneous injections may also traumatize the expanding skin, leading to a greater risk of complications.
A directional expander has been developed for breast reconstruction. Becker20 developed a permanent expander prosthesis for the same purpose. Manders and Friedman21 introduced a differential expander for the treatment of male pattern baldness. Despite many manufacturers’ modifications, the high technical standards for the devices have persisted. Their durability has been thoroughly tested, and their safety is well established.
Hallock22,23 was the first to study durability of expanders in a scientific way. He demonstrated in vitro that expanders can be overinflated at least 15 times the amount recommended by the manufacturer before rupture occurs. From in vivo studies, he concluded that expanders in the body could be safely overinflated to at least twice the amount recommended by the manufacturer without risk of rupture. However, the greater the pressure within the expander, the greater is the risk for leakage from the injection port. Nordstrom found that the average pressure necessary for leakage of the injection port is 32 mm Hg. This may explain reports of volume loss of saline injected into some expanders. To avoid such leakage, it is recommended to use the largest expander that can reasonably be inserted beneath the planned expansion area.
Expander Selection
When the shape of an expander is selected, the area to be reconstructed should be considered. For breast reconstruction, a round, pear-shaped, or directional expander is most helpful. For covering a large defect on the head or neck area, a rectangular expander is preferred. A crescent-shaped expander has been advocated for reconstruction of round defects. van Rappard et al24 compared the three most commonly used expander shapes (i.e., rectangular, round, and crescent) for their effectiveness. By mathematic calculations, rectangular expanders provided the most effective gain of surface area in expanded skin. In clinical situations, the gain in surface area compared with the area of the expander base is 38% for rectangular expanders, 32% for crescent-shaped expanders, and 25% for round expanders.24 Compared with conventionally shaped expanders, Brobmann and Huber25 found that less expansion is required to gain sufficient skin for repair when custom-made expanders are used for reconstruction of irregularly shaped defects.
In determining the appropriate size of an expander, Radovan26 initially suggested that the expander base be the same size as the defect to be reconstructed. It was thought that the surface area of expanded skin would be twice the area of the base of the expander used. However, with clinical experience, van Rappard demonstrated that only a fraction of the expected surface area is actually gained.24 Gibney27 recommended that the expander base be 2.3 to 3 times the surface area of the defect to be closed. This ratio of base size to expected increase in skin surface area is the generally accepted guideline and has been supported by van Rappard et al.24
In addition to the size of an expander, there are other factors that influence the amount of skin gained from expansion. These factors, described by Gibson6 and Sugihara et al,28 include the amount of undermining performed and the inherent extensibility of the skin. The response of skin to expansion differs according to the area of the body expanded and between individuals.