Congenital Melanocytic Nevi
Harvey Chim
Arun K. Gosain
OVERVIEW
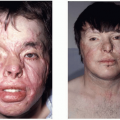
Congenital melanocytic nevi (CMN) are rare pigmented lesions that are believed to form between weeks 9 and 20 of gestation. Most are sporadic, but familial association is occasionally observed. Color ranges from light to dark brown and may appear blue in more darkly pigmented individuals. Lesions have well-defined borders, but vary considerably in size, pattern, and anatomic location. Although some lesions are flat, most cause some degree of skin surface distortion.
Small nevi are defined as those <1.5 cm in largest dimension, while medium-sized nevi are those between 1.5 and 19.9 cm. Giant nevi have most commonly been defined as those that are >20 cm in greatest dimension in adulthood. Because congenital nevi enlarge with overall growth, this 20-cm figure corresponds roughly to a 9-cm scalp or a 6-cm trunk lesion in an infant.1 Other definitions relating the lesion’s size compared with body surface area define a giant nevus as one covering more than 1% total body surface area in the head and neck or 2% or more body surface area elsewhere. Giant nevi have also variously been defined as lesions larger than 100 cm2, covering more than 5% total body surface area or a lesion that cannot be excised in a single surgery. As it is more difficult to determine the size of a lesion relative to the body surface area, the majority of authors report nevus size in terms of the greatest dimension of the lesion.
EPIDEMIOLOGY
Approximately 1% of the general population is thought to have CMN, most of which are of the small variety. The incidence of giant CMN is estimated at 1 in 20,000 live births.2 The very large “bathing trunk” examples are rarer still, occurring in approximately 1 in 500,000 live births.
EMBRYOLOGY, GENETICS, AND HISTOLOGY
Congenital nevi are theorized to represent a disruption of the normal growth, development, and migration of melanoblasts. Melanoblasts migrate from the neural crest to various sites in the body, including the skin, leptomeninges, eyes, and ears between the 8th and 10th weeks of gestation. These cells subsequently differentiate into dendritic melanocytes. Abnormalities in neuroectodermal development and arrested migration or differentiation of melanoblasts result in the formation of a CMN. CMN contains nevus cells and is present at birth or, in some cases, may appear within the first year of life. Nevus cells are distinguished from melanocytes by their lack of dendrites. The genetic etiology of CMN has been hypothesized to involve hepatic growth factor/scatter factor, which is involved in the migration and development of neuroectodermal cells and found in large amounts in CMN.
Characteristic histologic features of CMN include the following: (1) nevus cells within the middle to deep reticular dermis and subcutaneous tissue or deeper structures; (2) nevus cells extending between collagen bundles in the reticular dermis (“Indian” files) and around sebaceous glands, sweat glands, and hair follicles; (3) infiltration of arrector pili muscles by nevus cells; and (4) perifollicular and perivascular distribution of nevus cells resembling an inflammatory reaction. Small CMN may demonstrate dermal, junctional, or compound nevus patterns, with some specimens indistinguishable histologically from common acquired melanocytic nevi. In a giant CMN, the morphology of nevus cells can vary and is usually more complex. Several histologic patterns have been identified, such as compound or dermal nevus, blue nevus, neural nevus, and epithelioid cell nevus.
CLINICAL CHARACTERISTICS
CMN may appear initially as a hairless, pale brown flat lesion at birth, which evolves with time to develop variegation and hyperpigmentation. Dark, coarse hair may develop during the first 1 to 2 years of life. By 10 years of age, the lesions often develop a verrucous texture and become more elevated, with hypertrichosis and hyperkeratosis. Surface morphology varies widely and can be rugose, popular, pebbly, or even cerebriform appearance. Nodule formation typically represents benign neurotization in the nevus. CMN may be associated with multiple smaller satellite lesions dispersed over the trunk, extremities, or head and neck, with satellite lesions present in around 80% of giant CMN.
The most common anatomic location for a giant CMN is the posterior trunk, followed in frequency by the extremities and head and neck.3 Giant nevi may be found in specific anatomic patterns, such as the “bathing trunk” and “glove-stocking” distributions. Some interesting variants of CMN include the “kissing nevus,” occurring on adjacent aspects of the upper and lower eyelids, appearing as a single contiguous lesion when the eyelids are closed. This pattern suggests CMN development between the 9th and 20th weeks of gestation, where the eyelids are still fused.
The differential diagnosis for CMN includes other congenital pigmented lesions, such as epidermal nevus, nevus sebaceous, café au lait spot, and Mongolian spot. Other developmental anomalies may be associated with CMN, particularly of the giant size, such as spina bifida, scoliosis, elephantiasis, clubfoot, and cranial osseous hypertrophy. Patients with multiple small CMN should be distinguished from adults with multiple, acquired dysplastic nevi. This is an entirely different condition.
COMPLICATIONS OF CONGENITAL MELANOCYTIC NEVI
Malignant Transformation
When melanoma is reported, it tends to occur in the trunk3 and head and neck. It has never been reported in satellite lesions. Less than 0.5% of melanomas appear in preadolescent children, but 33% of those that do are thought to arise from CMN. Features that should prompt biopsy, if not complete early excision, include those suggestive of dysplasia or melanoma, such as ulceration, uneven pigmentation, bleeding, a change in shape, focal growth, or pain.
When considering malignant transformation, it is essential to distinguish between small and giant CMN. The lifetime risk of melanoma arising in small CMN has been reported to range between 0% and 5%7; however, in clinical practice malignant transformation is exceedingly rare in these small lesions. The risk in giant CMN has been reported to range between 0% and 42%.8,9,10,11 Two prospective studies reported a cumulative 5-year life-table risk of developing melanoma in giant CMN of 2.3%12 and 4.5%,1 respectively. The lifetime
risk was estimated to be at least 6.3%.13 Risk factors for the development of melanoma in CMN include large size (diameter > 20 cm), younger age (3 to 5 years), and multiple lesions (three or more). If melanoma does develop, the pattern differs between giant and small nevi. In giant CMN, melanoma usually develops deep to the dermal-epidermal junction or occurs extracutaneously (e.g., the central nervous system [CNS] or retroperitoneum) and is more difficult to detect. When malignant transformation occurs in small CMN, this tends to originate in the epidermis and often demonstrates a morphology similar to superficial spreading melanoma. Around 70% of melanomas in giant CMN occur by age 13, with 50% arising in the first 3 years of life, 10% later in childhood, and another 10% by puberty. Therefore surgical excision should be performed in early childhood. Transformation may occur later in life, and underscores the importance of long-term follow-up, even after surgical intervention. Other tumors with reported increased incidence in patients with large CMN include rhabdomyosarcoma, malignant cellular blue nevus, differentiated small round cell cancer, and spindle cell malignant tumor with lamellar differentiation.
risk was estimated to be at least 6.3%.13 Risk factors for the development of melanoma in CMN include large size (diameter > 20 cm), younger age (3 to 5 years), and multiple lesions (three or more). If melanoma does develop, the pattern differs between giant and small nevi. In giant CMN, melanoma usually develops deep to the dermal-epidermal junction or occurs extracutaneously (e.g., the central nervous system [CNS] or retroperitoneum) and is more difficult to detect. When malignant transformation occurs in small CMN, this tends to originate in the epidermis and often demonstrates a morphology similar to superficial spreading melanoma. Around 70% of melanomas in giant CMN occur by age 13, with 50% arising in the first 3 years of life, 10% later in childhood, and another 10% by puberty. Therefore surgical excision should be performed in early childhood. Transformation may occur later in life, and underscores the importance of long-term follow-up, even after surgical intervention. Other tumors with reported increased incidence in patients with large CMN include rhabdomyosarcoma, malignant cellular blue nevus, differentiated small round cell cancer, and spindle cell malignant tumor with lamellar differentiation.
Neurocutaneous Melanosis
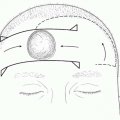
Large CMN may occur in the setting of neurocutaneous melanosis, in which collections of melanocytes are present in the leptomeninges. Malignant transformation can occur in this condition and result in primary CNS melanoma. Neurocutaneous melanosis is a result of dysregulation in proliferation and migration of melanoblasts in the CNS, resulting from an error in embryonic development. Even without malignant transformation, neurocutaneous melanosis can carry significant morbidity and mortality from seizures, hydrocephalus, cranial nerve palsies, developmental delay, and other signs of CNS irritation. Patients tend to manifest symptoms at two peaks in age. In infancy, patients present with hydrocephalus, increased intracranial pressure, or developmental delay. In the second to third decades of life, patients may have increased intracranial pressure or spinal cord compression. Neurocutaneous melanosis may also be asymptomatic. Risk factors for association of neurocutaneous melanosis include (1) CMN in the midline of the trunk or skull and (2) multiple satellite nevi (>20).
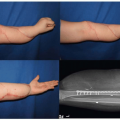
Magnetic resonance imaging (MRI) screening of the CNS is recommended for those patients who are at high risk for neurocutaneous melanosis. MRI should be performed early in life (between 4 and 6 months of age) prior to normal myelination of the brain, which will obscure visualization of deposits of melanin. Figure 20.1 shows a typical MRI of a patient with neurocutaneous melanosis. Cerebrospinal fluid cytology may also be useful to analyze for the presence of atypical malignant cells.
Treatment of symptomatic neurocutaneous melanosis may involve medical and surgical therapies such as placement of a ventriculoperitoneal shunt or radiation therapy. This condition typically carries a bad prognosis with death expected within 2 to 3 years of diagnosis. Treatment of asymptomatic neurocutaneous melanosis identified on MRI is more controversial and may best be done with serial MRI scans during childhood to follow progress and changes in lesions. In the absence of changing lesions, treatment may not be indicated.
MANAGEMENT
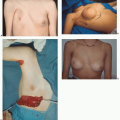
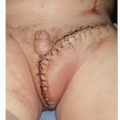
The fundamental guiding principle in the management of CMN relates to achieving a balance between treatment goals, namely, elimination (or at least reduction) of the risk of malignant transformation, preservation of function, and cosmetic appearance. At the same time, the risk of anesthesia and surgery remains a consideration. The risk of melanoma in giant CMN is well established. Intervention, if performed, should be done early in life, as the risk of malignant transformation is greatest in the first decade of life.14 Some authors have even suggested starting intervention at 6 months of age, with completion of staged surgeries before school age, so as to prevent stigmata associated with CMN and reconstruction.15 No clear consensus exists for management of small and medium CMN; however, it is apparent that the risk of developing melanoma is extremely low. Hence, recommendation would be for excision if performed, around puberty, with corresponding decreased risk of anesthesia. As a caveat, often the presence and location of the anticipated scar may in fact make excision of small CMN unwarranted.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree