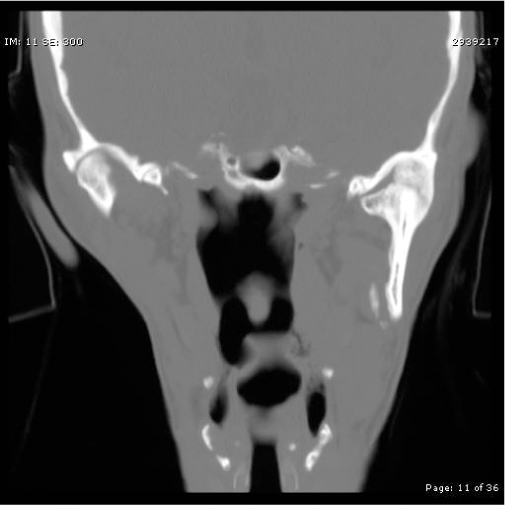
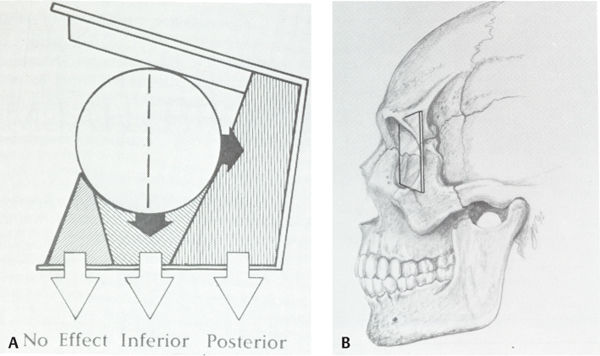
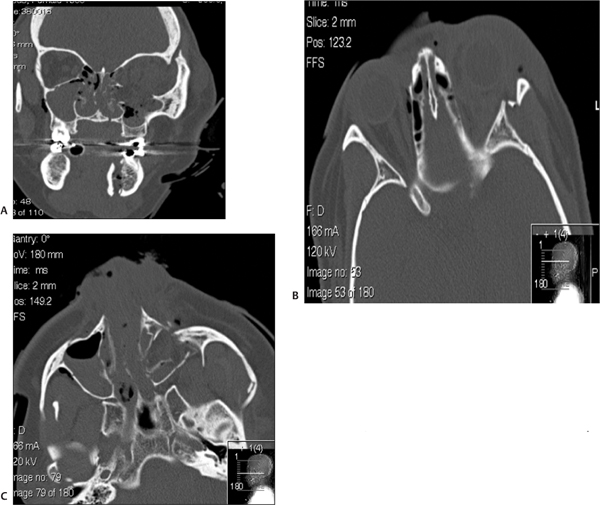
13 Facial trauma patients suffer injuries to complex soft-tissue structures as well as to components of the facial skeleton. Proper healing depends upon principles common to the management of facial trauma—that is, strict attention to soft-tissue closure techniques, patient health factors, meticulous wound care, and adequate reduction and fixation of fractures. The individual anatomic and functional features of each facial subsite, however, must be appreciated more fully to understand the development and management of complications. This chapter will address each of the major regions of the craniofacial skeleton separately—the mandible, orbit and zygoma, maxilla, naso-orbital ethmoid complex, and frontal sinus—as well as important facial soft-tissue structures. Infection is the most common adverse event following the treatment of mandibular fractures, with reported rates from 2.9% to 30% of cases.1–6 Reported rates of infection must be interpreted within the context of trends in treatment modalities. Rigid fixation techniques have assumed a primary role in fracture treatment in many medical centers in the United States over the past 20 years.3,7 Improved bone stability and reliability of healing with correct application of the techniques have resulted in significant improvements in the clinical outcomes in facial trauma. Thus, it is generally expected that rates of infection in the era of rigid fixation are less compared with rates in years past. Infection rates in clinical reports must also be judged based on the site of the mandibular fracture, the length of any prophylactic antibiotic course, patient compliance factors, and surgical technique.3,8,9 Infection may develop in instances of delayed treatment, patient comorbidities that result in immuno-compromise, technical errors resulting in improper reduction or stabilization (or both), and bacterial seeding of the fracture site, particularly in the presence of preexisting caries or dental infection.1,2,9–11 Infection in the fractured mandible often starts to develop when there is excessive movement of the ends of the fracture.1 Successful bone healing occurs two different ways, depending upon the mobility and interval distance of the fractured segments: healing and indirect bone healing. Complete immobilization with exact approximation of the fractured ends allows direct bone healing with minimal development of an intervening callus.1,12 When these conditions are not completely satisfied but the fractured ends are not distracted, the bone edges will experience some movement in the initial postinjury phase.1,12 Indirect bone healing commences in this situation. This process involves a cascade of events marked by the differentiation of hematoma to fibrocartilage and ultimately to bone. The formation of a callus occurs early in the process of indirect bone healing and serves as an early stabilizer of the bone segments and also as a foundation for the ingrowth of more specialized tissues.1,7,12 If there is a wide gap between the fracture segments, interfragmentary motion may be excessive, so a callus will not form.7,11 Excessive motion, which is often seen in delayed patient presentations or with the use of inappropriate stabilization techniques, promotes seeding of bacteria into the fracture1 and the ingrowth of fibrous tissue instead of cartilaginous and bone precursor cells.11,13 The initial choice of fracture treatment must be tailored to the fracture. Once biomechanical forces acting on the mandible were elucidated by pioneers such as Champy, Michelet, Luhr, and Spiessl, techniques for proper plate application followed.7,14 The ideal osteo-synthesis line (Fig. 13.1) described by Champy is based on an understanding of the three forces that act on the mandible during function: tensional, compressive, and torsional.7,14–16 When occlusal force is applied anteriorly, tensional forces are generated at the alveolar ridge and follow a line posteriorly along the oblique line. Tension tends to distract a fracture. Generally, compression occurs at the inferior border of the mandible during function, although forces applied in proximity to the fracture will often tend to compress the alveolar border and distract the inferior border.15,16 The suprahyoid muscles (digastric m., mylohyoid m., stylohyoid m., and geniohyoid m.) exert a torsional force on the anterior mandible (symphysis and parasymphyseal region), which requires that both the tension zone and the compression zone along the inferior border of the mandible be stabilized.15 Although the ideal osteosynthesis line originally described by Champy does not mandate the use of superior and inferior border plates for fixation of the body and angle, there is debate among surgeons regarding the ideal arrangement of fixation in these regions, given higher rates of infection in these areas of the mandible despite rigid fixation.3,17 Fig. 13.1 The ideal mandibular osteosynthesis line. This line, defined by Champy, marks the optimal location for the application of rigid fixation. Used with permission from Costantino PD and Wolpe M. Facial Plating Systems. In Papel ID, ed. Facial Plastic and Reconstructive Surgery. New York: Thieme; 2002:769–781. It is clear from historical outcomes that if the bio-mechanical forces and principles of osteosynthesis are respected, the application of rigid fixation will be successful most of the time. If, however, the hardware fails to achieve its goals, it becomes a foreign body and therefore an additional catalyst for infection. The physical findings in a patient with an infected mandible fracture include edema over the fracture site, pain, mobility of the fracture segments on palpation, purulent drainage, or hardware exposure.1 When we recall that most infected mandible fractures arise in the setting of fragmentary motion, it follows that treatment of this complication requires proper fixation of the mobile segments.1,18 Treatment calls for incision and drainage of abscess collections, removal of ineffective hardware, and the establishment of fracture immobility. Maxillomandibular fixation, stronger internal fixation, or an external fixator are all ways this can be achieved. Teeth in the fracture line are often extracted once an infection or healing delay has developed.1 Any devitalized tissue must also be debrided. Although it may seem counterintuitive to place hardware into an infected field, it must be remembered that the infection is not likely to resolve unless rigid fixation is established and that bone healing is jeopardized until stabilization is achieved.1,18 The use of antibiotics and minimization of patient comorbidities (e.g., malnutrition) certainly play adjunctive roles. Incidence of posttraumatic osteomyelitis is related to fracture infection. Whereas an infected fracture is considered a soft-tissue reaction to the stimulus of fracture mobility, posttraumatic osteomyelitis (PTOM) is a true bone infection. The clinical presentation includes severe pain and commonly an orocutaneous fistula with purulent discharge.19 These two entities will be distinguished radiographically by nuclear scans showing both an active inflammatory process in the bone as well as increased bone turnover.19 The treatment of PTOM has evolved in response to the availability of improved rigid fixation systems. PTOM may be managed in single or multiple stages. An open approach to the fracture allows the wide exposure required. Any failed hardware is removed. Wide bone debridement to healthy bleeding bone is undertaken. Load-bearing osteosynthesis is then applied in the form of a reconstruction plate. It is essential that the screws be placed in healthy bone. A suction drain is placed in the wound. If the amount of debridement required has resulted in a segmental defect, the decision must be made as to whether primary cancellous bone grafting will be performed. Alternatively, the patient may be placed on intravenous antibiotics and brought back for the bone graft after the infection has cleared. Kellman19 reported results of primary bone grafting and rigid fixation in 14 patients with PTOM. Initially, bone grafting was performed as a secondary procedure. Later in the clinical experience, primary plating and bone grafting were instituted. All of the patients went on to union with no further complications, confirming the safety of this approach. One factor that differentiates bone from many other tissues in the body is its ability to replace small gaps in tissue with bone instead of scar. As described earlier, bone ingrowth into the fracture site occurs by two processes, depending on the degree of movement at the fracture site. The growth factors and early cellular infiltrates that characterize a fracture hematoma are similar to those involved in soft-tissue wound healing. It is not surprising, then, that if osteogenesis is hampered in the early wound (increased fragmentary motion, infection, necrosis), bone healing is delayed and fibrous tissue ingrowth occurs by default. Certain conditions at the fracture site promote fibrous ingrowth over osteogenesis.13 The duration until bony union under optimal repair conditions has been defined as four weeks in children, six weeks in adults, and eight weeks in the elderly.12,13 These time frames were more clinically relevant when closed reduction was the primary modality for treating mandible fractures, because the time to healing determined the length of treatment. However, in the age of rigid fixation, the concept of delayed union takes more of a historical place. Delayed union results from incomplete healing caused by motion. When maxillary-mandibular fixation (MMF) is applied, incomplete healing due to motion can often be corrected by further stabilization.13 However, in the presence of a rigid fixation appliance, motion generally indicates failure of the fixation. Rather than MMF, correction generally requires removal of loose hardware, usually with the application of new, stronger fixation.1,3,12,20 Nonunion, in which the bone edges are in proximity but fibrous tissue has grown between the edges, can often be corrected by excising the intervening fibrous tissue that has bridged the bone gap and inserting proper rigid fixation, using a load-bearing repair.12,13,21 As mentioned previously, bone grafting may be necessary to bridge any gaps. Large volumes of cancellous bone can be harvested from the iliac crest or tibia.1,15 Rates of bone healing disturbance described as non-union have been reported in 1–3% of treated mandible fractures.12,18 Most patients in a review by Haug and Schwimmer18 had improper fracture stabilization, either because of flawed application of rigid fixation or loosening of closed fixation devices. Over half of their patient group developed infections. In Mathog’s series, 9 of 25 patients were identified as having poor fracture fixation.12 In both studies, the body of the mandible had the highest rate of nonunion or fibrous union, which confirms the role that weak stabilization plays, as this region of the mandible is subject to strong forces from the muscles of mastication. Malunion results when the bone fragments heal in a way that does not restore the premorbid occlusion. Although fractures of the parasymphyseal region are readily approached intraorally, the application of plates and screws to fractures in the posterior body and angle can be difficult transorally.15 If intraoral repair poses difficulty for the surgeon, there should be no hesitation about employing extraoral approaches to avoid compromising treatment. The surgeon must be meticulous about attaining adequate occlusion and must use information from the patient, past dental radiographs, dental models, photographs, and the patient’s wear facets to restore premorbid occlusion. Seemingly minor bony discrepancies can result in obvious changes in occlusion and thus an unsatisfactory outcome. Minor malocclusions can sometimes be treated with occlusal grinding,18 but more significant problems require orthodontic intervention22 and sometimes orthognathic surgery.23–25 The incidence and degree of sensory disturbance in the distribution of the inferior alveolar nerve (V3) can be related to the initial trauma or to subsequent surgical manipulation. The areas of the mandible through which the inferior alveolar nerve runs are most vulnerable and are the focus of most reports.26–28 Preoperatively, the degree of displacement of the fractured bone ends has consistently been shown to be correlated with rates of sensory deficits. Iizuka and Lindqvist27 reported that 73.5% of patients with greater than 5 mm of fragment displacement had hypesthesia based on pretreatment sensory testing, compared with 25.8% of patients in which the fracture was not displaced. Over all, 58% of their patients had hypesthesia immediately after the mandibular injury, with 46.6% reported to have some persistent sensory deficit after one year. In most of these cases, the residual deficit was mild, and many patients were subjectively unaware of significant hypesthesia. Patients who were edentulous had worse rates of recovery. Marchena et al.28 also published long-term follow-up data. The incidence of immediate postinjury sensory disturbance was 56%. They also found that the degree of fracture displacement was related to increased rates of postinjury hypesthesia. Objective testing revealed that only ⅓ of patients experienced full sensory recovery, though just 55% had awareness of the deficit. Widening of the lower face has been described as a complication after open reduction internal fixation of symphyseal fractures, particularly in the presence of subcondylar fracture(s) due to a failure to close the lingual cortical side of the fracture. This allows flaring of the mandibular angles, which results in the increased width. To prevent distraction of the lingual cortex, the plate should be slightly overbent at the line of the fracture to ensure compression of the lingual side. In the presence of bilateral subcondylar fractures, the angles should be pushed together to minimize this risk.29 Fractures of the condylar process of the mandible deserve individual consideration, because treatment goals for such injuries differ from those of injuries to other areas of the mandible. Successful outcome of treatment is judged primarily by the presence of unimpaired function of the temporomandibular joint (TMJ), whether or not reduction of the fracture is attained.1,30 With the high performance of titanium fixation plating systems and safer methods of approaching the TMJ,31 stricter criteria for successful treatment outcomes can be expected in the future, including restoration of posterior facial height and symmetric jaw movements, in addition to the attainment of premorbid occlusion.32,33 Clinically, unilateral condylar fractures can present with premature contact of the posterior dentition on the side of the fracture due to shortened ramus height, which generally occurs due to the overlap of the fractured fragments.34 The patient will therefore have a contralateral posterior open bite. The mandible deviates toward the fractured side because of unopposed action of the contralateral lateral pterygoid muscle (which is pulling the contralateral condylar process toward the midline).35 Bilateral condylar process fractures result in an anterior open bite (premature contact of the posterior dentition bilaterally), as well as retrognathia.30 It is important to consider initial treatment options, for certain types of therapy and aftercare may decrease complication rates. First, it should be pointed out that though many have advocated closed reduction using MMF, it is in fact a misnomer, as MMF does not result in reduction of the fracture, although it can restore the occlusion. For many years, closed reduction of condylar fractures was used almost exclusively. The main factors guiding this preference were functional adaptation at the joint, reasonably good occlusal outcomes, and the high complication rates experienced with open reduction, particularly the unsavory complication of facial nerve paralysis.18,30,34,36 Functional adaptation is the ability of the masticatory muscles to continue to function despite imperfect reduction of the condylar process after fracture.37 Since this in fact occurs, treatment of condylar fractures focuses on occlusal guidance.30 Training elastics attached to arch bars are applied so that the teeth are guided into occlusion. In many centers the standard treatment of the majority of condylar fractures has been and continues to be MMF for a short period (seven to fourteen days) followed by elastic MMF for occlusal guidance until the patient can attain a reproducible occlusion.18,34,36 The patient also begins jaw physiotherapy exercises after rigid MMF is released. This includes jaw-opening exercises and lateral excursive movements. Function, despite nonanatomical reduction, is critical to muscle adaptation and the prevention of TMJ ankylosis, trismus, and growth disturbances in pediatric patients.30,35,37,38 In our institutions, we believe that a period of rigid MMF actually delays the initiation of physiotherapy, so we prefer to progress immediately to training elastics and physiotherapy. Zide and Kent39 provided a classically accepted list of absolute and relative indications for open reduction of condylar fractures. The absolute indications include fracture dislocation of the condyle into the middle cranial fossa, lateral extracapsular dislocation of the condyle, an inability to restore occlusion with closed techniques, a foreign body within the TMJ, an open or penetrating injury to the TMJ, and a fracture that results in mechanical obstruction of TMJ. Open approaches to the TMJ include the submandibular approach (Risdon), the preauricular approach, the retromandibular approach, and the intraoral approach, which may be performed with endoscopic guidance.15,30,31,34 Stated advantages of open reduction and fixation include restoration of ramus height, avoidance of facial asymmetry, the possibility of immediate function and superior occlusal results found by some authors, even when absolute indications are not present.31–34 Few surgeons open fractures of the condylar head, though this may become more common in the future as well.34 Several reports have evaluated the development of malocclusion in groups of patients treated with MMF versus open reduction and internal fixation. Worssae and Thorn40 found that 8 of 24 (30%) of patients treated with MMF developed malocclusion, compared to 1 of 28 patients who had the joint opened. Ellis et al.36 found that at one year follow-up, patients who had closed treatment were consistently rated to have significantly higher percentages of poor occlusions compared to cases treated with ORIF. Malocclusion may be the result of poor reduction of the fracture segments with persistent ipsilateral premature contact, or it may be the result of growth interference in a pediatric fracture. The condylar head can partially resorb over time as a response to the initial injury or from the surgical manipulation required during treatment.37,41 The malocclusion in these instances is caused by a shortened vertical ramus height on the fractured side. For less severe malocclusions, orthodontics may be adequate treatment.34 Orthognathic surgery can correct the malocclusion by using osteotomies to reposition the proximal ramus and restore the ramus height in more severe cases.24,41 This is a successful approach for unilateral cases. A vertical ramus osteotomy allows the proximal segment to be positioned superiorly to equalize the ramus heights. If anterior or posterior repositioning is required, a sagittal split osteotomy is performed.41 When planning orthognathic surgery to correct traumatic dental-skeletal anomalies, the surgeon must use an approach that not only corrects malocclusion but also repositions the bone fragments in a stable manner to avoid relapse. This may mean performing osteotomies away from the site of the initial injury.42 Superior movements and clockwise rotation of the mandibular ramus are stable, while inferior positioning of the ramus and counterclockwise rotation are unfavorable.43 Although one option to correct an anterior open bite would involve bilateral mandibular ramus osteotomies, the resulting counterclockwise rotation of the distal segments required to restore occlusion is a situation prone to relapse. Alternatively, the anterior open bite may be corrected by a LeFort I osteotomy with posterior impaction of the maxilla.23 This allows autorotation of the mandible superiorly with closure of the open bite. When condylar/subcondylar fracture patients are treated with MMF, the premorbid condyle position is not always achieved. Often the condylar process is displaced in such a way that the fracture segments overlap, or the condyle may not be positioned upright. Either of these situations shortens the total height of the posterior mandible and may result in decreased posterior facial height and facial asymmetry.32,34 In a study of posterior facial heights of patients undergoing closed or open reduction, Ellis32 found a statistically significant difference between the fractured and nonfractured sides in patients treated with closed reduction (mean of 5 mm decreased posterior facial height at two years follow-up). Most patients achieved functional occlusion; however, their facial appearance was not commented upon, so it is not clear how directly the change in ramus height translated to obvious facial asymmetry. TMJ ankylosis occurs when the joint space is partially or totally obliterated by fibrous or osseous tissue. Patients with unilateral ankylosis will have trismus and deviation to the affected side. Reviews of TMJ ankylosis implicate trauma as the cause in 30–98% of cases.44–46 Although the pathophysiology of ankylosis is not completely understood, several factors are known to play a significant role. Bone growth is enhanced in the presence of hematomas and growth factors released by platelets within clots. This is felt to be particularly important in pediatric condylar fractures, because the condylar head is highly vascular.30,37,47 In addition, damage to the meniscus and joint dislocation37 have been correlated with increased rates of ankylosis. Prolonged joint immobilization during extended courses of rigid MMF, combined with inadequate adherence to a protocol of jaw motion exercises, results in decreased TMJ function during convalescence. These factors predispose the patient to the development of ankylosis, although ankylosis can still develop despite mobilization. When a patient with traumatic TMJ ankylosis is encountered, evaluation requires a maxillofacial CT scan to assess the full extent of the lesion (Fig. 13.2). As pointed out by Raveh,47 the bony overgrowth can extend beyond the glenoid fossa to the adjacent middle cranial fossa and medially along the skull base. Surgery is indicated to improve the maximal interincisal opening (MIO), restore joint function, and prevent future growth disturbance in pediatric patients.44,45,47 Many surgical approaches have been described. All of the described protocols advocate safe but aggressive removal of the bony or fibrous lesion. In a series of 14 patients (both pediatric and adult), Kaban et al.44 reported retrospective data with a treatment protocol that included resection of all abnormal bone, unilateral or bilateral coronoidectomy to augment mobility, an autologous costochondral graft to replace the condyle, and lining of the glenoid fossa with a turned-down temporalis muscle flap. All patients were placed in MMF for a brief time and then enrolled in jaw physiotherapy for one year. Other surgical approaches call for lining the TMJ with auricular cartilage or total joint replacement with an allopastic joint.30,46,47 Fig. 13.2 Coronal CT scan of left temporomandibular joint ankylosis. Growth disturbance of the condyle highlights the importance of restoring normal joint function in the pediatric population.35,47,48 It has been confirmed by human and animal studies that fractures heal more quickly in younger patients.30 Although functional adaptation is the rule in restoration of joint function in adults, young children usually experience joint remodeling and recapitulation of the premorbid condyle and fossa anatomy after treatment.44 The development of scar tissue can tether the downward growth of the condyle and ramus if early treatment and therapy are not instituted. Facial stigma include a shortened ramus on the fractured side, deviation of the chin toward the fractured side, and flattening of the face on the contralateral side.30,48 Dental compensations lead to a posterior crossbite on the affected side. Condylar fractures can be difficult to diagnose in children if the evaluating physician does not have a high clinical suspicion. The examination may be limited, especially if the child is anxious. The condylar fracture may be difficult to detect by plain films (mandible series), since there is a higher percentage of green-stick fractures in children.30 It is recommended that a condylar fracture be suspected in children with trauma to the chin and conclusively ruled out.30 Distraction osteogenesis is being used successfully in the treatment of the hypoplastic ramus in these patients.48 Fractures of the facial bones around the eye can be associated with globe malpositions. Enophthalmos is defined as posterior displacement of the globe, while hypoglobus refers to inferior displacement of the globe.49–51 Increased orbital volume caused by trauma is the most frequent cause of enophthalmos and hypophthalmos. Other factors contributing to the development of enophthalmos include tethering of orbital contents within an unrepaired fracture and fibrosis and scar contracture of intraorbital contents.51–55 Atrophy of intraorbital fat has been, for the most part, discounted as a significant factor, although actual loss of orbital fat certainly can be.54 Enophthalmos results from fractures of the orbital walls behind the axis of the globe (Fig. 13.3), which includes all of the lateral wall, most of the medial wall, and the posterior aspects of the inferior and superior walls.54,56 Hypoglobus results from bone loss from the floor at the globe axis.54,56 Bone loss anterior to the globe axis does not generally affect globe position56 (Fig. 13.3). Facial trauma surgeons usually encounter enophthalmos as a delayed complication of orbital blowout and zygomatic fractures, since only rather severe fractures will present with acute enophthalmos.49,52,57–59 Patients with unrepaired fractures and orbital volume increases will typically develop enophthalmos weeks after the injury, once edema has resolved.57,59,60 With CT scan imaging now a routine part of the work-up in patients with facial trauma, large-volume fractures are detected immediately.57,58 The goal of early fracture management is to predict, based on CT assessment of the fractures, which patients are likely to develop enophthalmos.57 Many surgeons advocate treating high-risk fractures within one to two weeks of the injury, at a point when edema has resolved but before fibrosis has developed.57–59,61 There have been strong advocates for a more expectant posture whose justification relied on a relatively low rate of postinjury enophathlmos and successful camouflage treatment when it did arise;62 however, this management philosophy is not commonly accepted today. Currently, a large-volume fracture (50% or more of the orbital wall involved) is a clear indication for exploration and repair.57 Posttraumatic enophthalmos is due to orbital blowout fractures and displaced zygomatic fractures.54 Patients presenting with late enophthalmos generally fall into two groups: those who were not treated primarily and those who have had prior surgical repair without full restoration of the orbital volume present before the injury.49,54,60,61 Three to twelve percent of repaired zygomatico-maxillary complex (ZMC) fractures may be complicated by enophthalmos.63–66 Hawes and Dortzbach found that while 7% of patients who had repair of the orbital fracture within two months of the injury developed enophthalmos, 88% of patients presenting for treatment two months after their injury had enophthalmos.59 Catone et al. presented their data on 20 patients with unrepaired orbital blowout fractures, 15% of whom developed enophthalmos.58 The zygoma makes up the inferolateral orbit through three of its five projections67 (Fig. 13.4). Volume-enhancing vectors of movement include lateral displacement of the zygoma and inferior distraction exacerbated by the pull of the masseter muscle.56,63 The zygoma contributes significantly to facial form, and its posterior displacement leads to a flattened malar prominence and increased facial width from outward bowing of the zygomatic arch54,61 (Fig. 13.4). Some important technical errors can lead to failures of primary repair of orbitozygomatic fractures. Adequate dissection into the posterior orbit is required to identify the posterior ledge of the fracture, which may extend 40 mm posterior to the infraorbital rim.54,67 Exposure of the medial orbital wall must be addressed when required, and this wall is sometimes overlooked.54,56,68 The key surgical principle in the treatment of zygomatico-maxillary complex (ZMC) fractures is three-point reduction of the zygoma and at least two-point fixation.56,63,67 Two-point reduction does not ensure that the zygoma is not rotated at another articulation.56,63,67 Persistent diplopia may occur in patients after timely primary fracture repairs. Diplopia in the acute setting has several causes, which can be broadly categorized as mechanical or neurogenic. Extraocular muscle entrapment, edema, or hemorrhage (either in combination or isolation) may contribute to mechanical restriction of globe motion. Injury to the motor nerve of an extraocular muscle represents a neurologic cause of diplopia after orbitozygomatic trauma.58,68 As might be expected, postoperative resolution of diplopia is variable, depending on its cause. Edema should resolve with a gradual improvement of diplopia over one to two weeks.57 The course of recovery of diplopia from a neurogenic cause is harder to predict and depends on the degree of nerve injury. Prolapse of orbital contents can result in both enophthalmos (delayed) and diplopia and explain why these symptoms often coexist.54,68 The goals of treatment in this instance are volume restoration with globe repositioning and improved extraocular muscle function after it is released from the constraint of the fracture.54–56,60,68 The increased use of endoscopic approaches to the repair of orbital blow-out fractures offers several advantages in terms of visualization and avoidance of a lower lid incision. Application in the right cases and recognition of the limitations of this approach are important factors in adequate repair with the endoscopic technique. Endoscopy via the transantral route also has a role as an adjunct to standard eyelid approaches to confirm complete reduction of the orbital contents and proper placement of floor implants.69 Several aspects of the residual injury can be quantified. Zygomatic fractures may present with all of these secondary findings, while orbital blowout fractures are less often associated with the facial deformities outlined below. Posterior globe displacement is well visualized from the worm’s eye or bird’s eye view. A Hertel exophthalmometer quantifies globe projection by measuring the projection of the orbit relative to the lateral orbital rim.49,54,60 Any difference greater than 3 mm between the globes is abnormal,49,68 though differences of 1–2 mm may be noticeable on some people. When the lateral orbital rim is displaced (as may be the case in a ZMC fracture) a Naugle exophthalmometer is used—the reference point is the forehead.54
Complications of Facial Trauma Repair
 Mandibular Fractures
Mandibular Fractures
 Condylar and Subcondylar Fractures
Condylar and Subcondylar Fractures
 Orbit and Zygoma Fractures
Orbit and Zygoma Fractures
Malar Flattening and Increased Facial Width
Diplopia
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree