3 Intradermal and hypodermal injections have become a multimillion dollar industry in contemporary cosmetic medicine. Minimally invasive facial rejuvenation techniques, such as soft-tissue augmentation with dermal fillers and rhytid effacement with botulinum neurotoxin type A (BT-A), have exponentially increased in popularity. This increase in popularity and demand is due to a combination of fast results with minimal recovery time as well as excellent product safety records. As more patients seek cosmetic and aesthetic improvement without surgery, a review of the potential complications that can occur from these minimally invasive techniques can be advantageous. By reviewing complications associated with the most often used injectables, both the physician and the patient will become better informed of both potential complications and expected results. This chapter will discuss BT-A, review its associated complications, outline the most commonly used injectable fillers, and briefly discuss the potential complications associated with each product: collagens, hyaluronic acids, Radiesse, Sculptra, and synthetic injectables. Botulinum toxins have been used successfully to manage facial aging by effacing rhytides and hyperkinetic lines of the face and neck. Although seven different serotypes of botulinum toxin are found in nature (A through G), serotype A is the most commonly used [Botox and Botox Cosmetic (Onabotulinum toxin A), Allergan, Inc., Irvine CA. Dysport (Abobotulinum toxin A), Medicis Aesthetics, Inc., Scottsdale, AZ. Xeomin (Incobotulinum toxin A), Merz Aesthetics, Inc., San Mateo, CA]. Each of the serotypes is defined by its specific biologic action, specifically the cleaving of different proteins involved in active transport of acetylcholine into the neurosynaptic cleft responsible for muscle contraction. Relaxing the muscle leads to effacement of rhytides, causing an improved cosmetic appearance. Although extensive research and data have been collected to support the safety of BT-A in therapeutic doses, it is important to note that complications can occur. Complications related to BT-A are usually minimal, short-lived, and reversible. The majority of complications can be prevented with proper injection techniques, correct dosing, and a thorough knowledge of facial anatomy. Bruising, pain, and edema can occur from BT-A injections; however, it should be noted that they are usually associated with the injection itself and are not complications of the neurotoxin. Mild bruising has been reported in as many as 11% to 25% of patients who received Botox injections.4 Bruising is increased in patients taking nonsteroidal anti-inflammatory drugs, aspirin, anticoagulant medications, vitamin E, or large doses of ginseng, garlic, and ginkgo. Patients should be advised to discontinue taking these products 7 to 10 days before treatment if medically possible. Injection site pain is an adverse event associated with any injection, but it can be minimized with the use of a topical lidocaine (Topicaine, ESBA Laboratories, Mountain View, CA) and benzocaine, lidocaine, tetracaine (BLT, TLC Pharmacy, Hermosa Beach, CA). Both pain and edema can each be minimized by applying ice to the injection sites directly after injection. The use of 32-gauge needles will also help to minimize bruising and discomfort. Immune responses to BT-A have occurred in patients receiving local injections of botulinum toxin (BTX). Development of antibodies and T lymphocytes primed to epitopes on the heavy chain of the toxin has been reported.4 Loss of action can occur in patients who have received one or more previous BTX injections; upon a subsequent injection, the patient does not respond to treatment. Although BT-A resistance occurs in only 5% of patients, it is an important factor to consider.1 This immunogenicity is presumed to be caused by the formation of serum antibodies.4 The antibodies formed against BT-A can block the activity of the neurotoxin by blocking the presynaptic terminals, thereby allowing the release of acetylcholine and leaving the patients as nonresponders. Resistance can be measured using the Frontalis Type A Antibody Test (FTAT), where patients are injected with 15–20 units of BT-A in the frontalis muscle. The patient is considered a nonresponder or resistant if the eyebrows can be raised after two weeks.2 The immunogenicity of BT-A is thought to be dependent on the amount used, the duration between injections, and the amount of protein surrounding the toxin. It has been noted that repeat treatments with large doses may increase the likelihood of developing antibodies.3 Immune response can be minimized by using the smallest effective dose and by increasing the amount of time between injections. BT-A has a large therapeutic window, which increases the possibility of overtreatment. As a simple rule, the minimal effective dose should be used to give maximum efficacy with a lower risk of developing immunogenicity. Because the protein content may be proportional to antigenicity, Botox has released recent batches with lower protein content in the hope of decreasing immunogenicity.3 Dysport, another serotype A toxin, was recently approved by the FDA and has a lower protein load.4 In patients who have demonstrated nonresponsiveness, different serotypes of the botulinum neurotoxin may result in different antibody responses, thus serotype B (Myobloc) may be an option for these patients. BT-A is commonly injected into the frontalis muscle to reduce transverse forehead rhytides. However, over-treatment of the frontalis muscle can produce brow ptosis, a complication resulting in a drop of the brow below the supraorbital rim. Brow ptosis can be easily avoided by reviewing facial anatomy and using correct injection techniques, as well as adhering to patient selection criteria. The frontalis muscle lies beneath a thick layer of sebaceous skin and subcutaneous tissue. The muscle originates on the galea aponeurotic layer superiorly and interdigitates with the procerus, corrugator and orbicularis oculi muscles that overlie the brow region. Successful rejuvenation of the rhytides requires some relaxation of the frontalis muscles, but not enough to cause subsequent brow ptosis through the unopposed drepressor activity of the procerus, corrugator and orbicularis oculi muscles. Deep injections into the muscle are performed in a staggered distribution, with ~1 cm distance between injections positioned at least 1.5 cm to 2.0 cm above the brow margin. If injections stay above the brow, subsequent ptosis and brow asymmetry are avoided. Correct patient selection is also an important consideration to avoid brow ptosis. Patients with heavy, thick brows should be avoided, as well as patients who had brow ptosis before receiving botulinum toxin. Bepharaptosis (eyelid) ptosis is defined as inferior malposition of the upper eyelids; it can occur from the use of BT-A and most commonly occurs when BT-A is injected into the orbicularis oculi, corrugator supercilii and procerus muscles5 (Fig. 3.1). Eyelid ptosis usually occurs from either incorrect placement of BT-A or diffusion of the toxin through the orbital septum to the levator palpebrae superioris muscle. The onset of ptosis can range from 48 hours up to 14 days after the injection and can last for two to four weeks. Blepharoptosis is usually subtle; however it can become exaggerated at the end of the day or when patients feel tired. Ptosis can very easily be avoided by advising patients to avoid manipulation and to remain in the upright position for 3 to 4 hours after the injection. Injections to reduce glabellar rhytides should not be performed lateral to the midpupillary line. Fig. 3.1 Patient with eyelid ptosis of left lid. Fig. 3.2 Patient with eyelid ptosis of left lid. Note attempted frontalis compensation (elevated left eyebrow). If ptosis occurs, the use of apraclonidine eye drops, 0.5% (Iopidine, Alcon Laboratories, Forth Worth, TX) can be an effective therapy.6 Apraclonidine is an α2-adrenergic agonist, causing contraction of the Müller muscles and a resulting elevation of the eyelid. One or two drops should be applied three times daily until ptosis resolves. It is important to note that contact dermatitis has been noted as a potential complication with use of apraclonidine.7 Systemic complications are not an issue when botulinum toxin is used in small quantities with standard preparations. There are, however, reports that botulinum toxin has been detected in the hypothalamus after intradermal injection, which would indicate it travels systemically and even crosses the blood-brain barrier. There have also been misadventures with treated individuals contracting systemic toxicity (botulism) after injections of botulinum toxin when non-standard preparations and large doses were used. It is imperative, therefore, that physicians who treat patients with botulinum toxin use only the recommended doses of FDA-approved toxins. Whatever the actual mechanism, the lack of deleterious systemic effects with these simple guidelines implies that while research into BT-A mechanisms continues, physicians can safely use BTX for therapy.8 Throughout the aging process, elasticity and collagen levels decrease, creating wrinkles in a fairly predictable manner. As a corrective measure, dermal fillers offer a successful method for soft-tissue augmentation and facial rejuvenation without the risks associated with surgery. Thus, as these products gain popularity and more products emerge, it becomes increasingly important to discuss the differences between the products and the complications involved with each. For all dermal fillers, the potential complications can be divided into either early or late complications. Early complications, which can be seen with all fillers, include bruising, erythema, and edema. Late complications are usually due to an immunological response or an incorrect injection technique. The following sections thoroughly discuss the potential complications associated with each filler and review strategies to avoid and treat adverse events, so complications can be minimized and hopefully avoided.
Complications in the Cosmetic Office Setting
 Botulinum Toxin
Botulinum Toxin
Potential Complications with BT-A Injections
Bruising, Pain, and Edema
Immunogenicity
Brow Ptosis
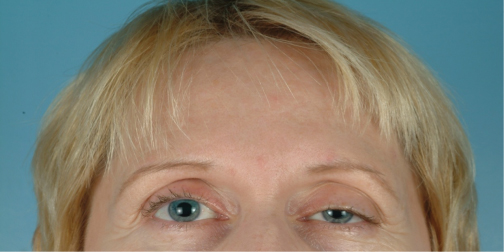
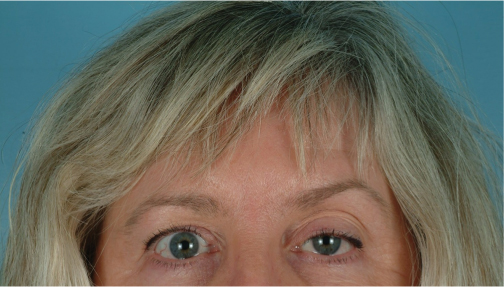
Blepharoptosis (Figs. 3.1, 3.2)
Systemic Complications of BT-A
 Dermal Fillers
Dermal Fillers
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree