PRODUCTS: METHODS AND INSTRUMENTATIONS
Author
Iqbal Sadiq, M.Phil.
Director Research and Technology
Product Investigations, Inc.
151 East Tenth Ave., Conshohocken, PA 19428, USA
ABSTRACT
In the field of skin research, visual examination and touch are no longer solely employed to assess skin conditions. The field has evolved to include instrumental methodologies to quantitatively and qualitatively assess skin properties. Principles of physics, chemistry, biology, and engineering are combined to study skin both at the surface and in-depth.
Cosmetics and Skincare products are used to enhance the look and feel of the skin and to protect the body from the elements of nature. To study the effects of cosmetics and skin care products on skin, biophysical measuring techniques can be employed in a variety of experimental models. Both dose-response and time-response studies can be performed with greater precision. For example, topical products may affect skin in a variety of ways (e.g., changes in stratum corneum hydration, trans-epidermal water loss, blood flow, pigment, morphology and elasticity, etc. These changes are measured by different instruments.
This chapter presents an overview of some of the commonly used, powerful techniques currently in use. The electrical impedance of skin has been shown to change with stratum corneum hydration and, based on this principle, a number of devices are available that are routinely used in studying stratum corneum hydration changes. The skin barrier can be studied by trans-epidermal water loss measuring devices. To quantify erythema on skin a number of devices based on various physical principles are in use (e.g., colorimetry, spectrophotometry, and laser doppler flowmetry).
Many imaging techniques have been developed to reveal subtle changes in skin on the surface and in depth. High-resolution digital photography with options of polarized and fluorescence imaging and videomicroscopy are useful to explore the microstructure. Some more advanced techniques such as in vivo laser confocal microscopy and optical coherence tomography reveal the horizontal and vertical sections of skin at various depths. Measuring skin topography parameters is useful to show smoothing of skin surface after a product application. We can now study viscoelastic properties of skin in a variety of ways using twisting, impacting, suction, and shearing devices. The list of skin measuring tools is growing . . .
11.5.2 Cosmetics and Skin Care products for human use
11.5.6 Trans-Epidermal Water Loss
11.5.7 Skin Blood Flow, Color, Erythema
c. Reflectance Spectrophotometry
2. Polarized Light Photography
4. Ultraviolet Light Photography
e. Optical Coherence Tomography (OCT)
b. Phase Shift Fringe Projection Device
c. Calculation of Roughness Values
11.5.10 Viscoelastic Measurements
11.5.11 Some Ex Vivo Techniques
b. Cyanoacrylate Surface Biopsy
11.5.12 Application of Bio-Instrumentation: Some Examples
The effect of skin care products that improve the structure and function of skin, as well as enhance the appearance of skin, can be studied using a number of bioengineering techniques. We no longer depend solely on clinical gradings. The idea is to get a complete picture of improvements, not just look at one or two parameters. Traditionally, visual examination and some simple evaluations have been used on large pools of subjects to show the efficacy of these products. Often the difference between the treatment and control is subtle, requiring advanced methodologies to be employed. A good collection of these skin-measuring techniques are given in Handbook of NON-INVASIVE METHODS and the SKIN, second edition [1]. Some skin-imaging techniques are given in BIOENGINEERING OF THE SKIN, Skin Imaging and Analysis, second edition [2].
Measuring stratum corneum hydration using electrical devices is a commonly used method. Often there is a need to use advanced methods like measuring skin hydration kinetics which are based on the property of stratum corneum to hold, release or accumulate water. These include sorption-desorption (S-D) and moisture accumulation test (MAT). The use of sorption-desorption method is described by Tagami et al. [3] and moisture accumulation method by Pellacani and Seidenari [4].
The barrier function of SC can be assessed by measuring the trans-epidermal water loss (TEWL) as well as by using a number of chemical probes, e.g., methyl-nicotinate, lactic acid, and balsum of Peru. Furthermore, penetration of fluorescent dyes, e.g., pyranine, can also be utilized.
Erythema can be assessed in a variety of ways. The erythema may be graded visually; however, it will depend on the particular observer, ambient light, previously seen colors as well as the mood and prejudices of the observer. Human eye is good in discriminating colors and brightness of objects when they are adjacent, but poor in grading single color or intensity. It also cannot remember a previously seen color or intensity quantitatively. The search for an objective method to assess erythema, over the last 100 years, produced a range of techniques. Topical drugs and skin care products are tested by either comparing post-treatment erythema values to pre-treatment or comparing the erythema value on the test site to the adjacent normal skin. Conversely, some products cause vasoconstriction, resulting in an apparent blanching. The pallor is caused by relatively lower amount of blood at the test site. Both redness and blanching can be graded visually as well as measured by various instrumental modalities, e.g., visual examination, various scales, graded colored paper, photoplethysmography, colorimetry, diffuse reflectance spectrophotometry, blood flow by laser doppler imager, digital color photography, thermography, etc. Subclinical erythema can be detected by reflectance spectrophotometry. Laser doppler imager is not useful for blanching assays. Blanching (by corticosteroids) can be detected very well by reflectance spectrophotometry.
Skin pigmentation can be measured by a variety of techniques, some of them common to erythema measurement, e.g., visual examination, various scales, colorimetry, reflectance spectrophotometry, optically filtered digital photography, etc. For melanin pigmentation we can also add controlled UV-photography.
Optics and advanced imaging techniques are important in showing the effects of products on skin. Filtered digital photographic techniques (cross-polarized, parallel-polarized, and fluorescence) can reveal features of skin that are not shown in standard photography. Ultraviolet light photography dramatically reveal the pigmented lesions of skin. Side-lighted videomicroscopy dramatically enhances the view of scaliness of skin, especially if blue-UV light is used for illumination. This method also shows an enhanced view of the pigmented lesion. Ultrasonography shows the skin structure (cross-section) and reveals the presence of edema. Optical Coherence Tomography (OCT) shows cross-section of skin at a much higher resolution than ultrasound. Thickness changes in SC and epidermis can be measured by Optical Coherence Tomography (OCT). Some applications of OCT on skin are given by Pagnoni et al. [5]. Confocal Scanning Laser Microscopy reveals cellular structure in various strata of skin as horizontal sections, complementing OCT’s cross-sectional view. The confocal microscope can also be used to measure thickness of stratum corneum and epidermis. Confocal microscope images show melanin-rich areas as bright structures, particularly near basal layer. Rajadhyaksha et al. describe a confocal microscope in detail [6].
An important parameter in studying skin is topography. In the past, replicas of skin have been used to assess the surface for roughness and glyphic line structure. The replica samples have been imaged using many different angles of illumination. In some experiments we have used a narrow-angle (~ 10°) illumination by a ring light to enhance the fine surface structure. The image is digitally captured and analyzed using computer programs developed in our laboratory. The line structure is analyzed for lengths and areas. Also surface roughness is calculated. The fringe projection device has, by and large, replaced the replica method. Fringe-projection device with phase-shift procedures can reveal ultra-fine changes in skin surface roughness. This device images skin surface directly and the calculation of topography parameters are much more precise. Skin roughness and smoothness values can be calculated in a variety of ways. We have shown the disruption of surface in the photo-damaged skin compared to relatively normal skin. Detailed descriptions of the fringe projection device are given by Jasper et al. [7] and Frankowski et al. [8].
Viscoelastic properties of skin are important for a healthy skin. Skin is a complex composite made up of elastic and viscous parts. The bulk of elasticity in skin resides in the dermis with its collagen and elastic fiber matrix. The epidermis and stratum corneum, however, also influence the elastic properties somewhat. In general, any product that affects the hydration levels of skin or influences the dermal fiber network would show change in the viscoelastic properties of skin. To measure the elastic properties, a force (stress) is applied to the skin and then released while deformation (strain) is measured during this cycle. The in vivo elastic properties of the skin can be measured by several methods like ballistometry, dynamometry, torque, and suction devices.
Some ex vivo techniques are available to quantify features of skin, e.g., sebum output, number and size of follicular casts, and desquamation. Samples are first collected off the skin by an appropriate method and then an image is obtained under a microscope. Image analysis programs are then used for quantification.
The desquamation property of skin is commonly studied by collecting the surface scales on an adhesive disc. A more advanced method is to harvest 3D squame samples sequentially from the same site and look at the dynamics of scale-collection.
Many of the methods described above have been used to study sun-damaged skin, also referred to as photo-damage. There is great interest in treating the signs and symptoms of sun-damage. The basic science of skin intrinsic aging and photo-aging have been described by many authors [9], [10], [11], [12].
11.5.2 COSMETICS AND SKIN CARE
PRODUCTS FOR HUMAN USE
Cosmetics and skin care products are used to enhance the look and feel of the skin and to protect the body from the elements of nature. Topical drugs, on the other hand, are used to prevent or treat skin diseases. Considerable overlap, however, occurs between these two fields; e.g., control and treatment of dry skin are tackled by both skin care products and skin pharmaceuticals.
The development of a skin care product starts with market research and current fashion trends; a product is then designed based on technical research and development. Before the product reaches the consumer, a number of testing and research procedures are followed at various steps. First, chemical and microbiological stability is tested. Safety tests are then performed followed by efficacy testing. Finally, usage testing is done, which is based on sensory, tactile, and visual appeal of the product.
The efficacy testing of a product on human subjects starts with hypotheses. A good hypothesis is based on current knowledge of the properties of a product and its component chemicals, and the potential effect on human skin. The experiment is designed by choosing appropriate measuring techniques and using currently acceptable principles of statistics. The size of the test panel is based on the precision and resolution of measurements. Proper controls should be used to show that the active ingredient, indeed, have produced the change.
The test product should be blinded from the subjects as well as from the evaluators to rule out any bias that may creep in. In general, a good test would have double-blinded, randomized, and placebo-controlled design. Negative and positive controls may also be added.
Human skin covers the whole body, an approximate surface area of 2 m2, varying in thickness with location as well as with age, sex, ethnicity, and dermatological conditions of health and disease.
The skin is divided into two main compartments: the outer epidermis and the inner, and much thicker, dermis. As we journey from the outermost layer of skin to the innermost, we pass through various strata of epidermis and dermis. A sketch of skin strata is shown in Figure 1.
The outermost surface is the stratum corneum (SC), a multilayered, keratinized surface with flattened and non-nucleated cells, post mortem. This is followed by stratum granulosum (SG), which is composed of one or more layers of granular cells that contain keratin fibers and either has shriveled nuclei or no nuclei. Below the SG there is a thick layer, stratum spinosum (SS), composed of several layers of cells with large oval nuclei and spiny processes. This is followed by stratum basale (SB), which is the last epidermal layer at the dermal-epidermal junction. The SB is composed of a single layer of columnar cells (basal cells), which include melanocytes and Merkel cells. The melanocytes are dendritic cells that perform melanogenesis through melanosomes. The melanin pigment is finally transferred to the keratinocytes. At the basal layer, cells divide continuously and move outwards towards the surface of the epidermis.
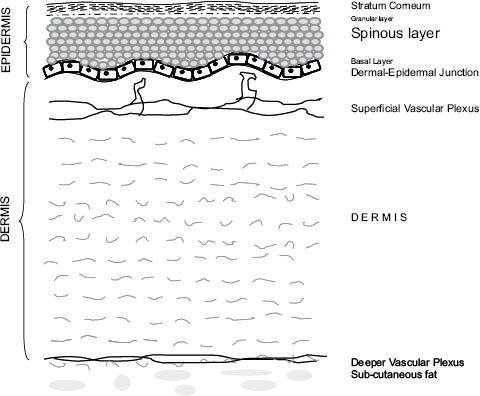
Figure 1: A general sketch showing the various strata of skin.
The dermal-epidermal junction is a wavy surface forming peaks and valleys (rete ridges). On the dermal side of this papillary surface, is the skin blood microvasculature.
The dermis forms the bulk of the skin, protecting the body from physical injury, providing pliability, elasticity, and tensile strength. The dermis is composed of bundles of fibers of various types (e.g., collagen and elastic fibers), distributed among which are blood vessels and nerves. Dispersed among these are hair follicles, sebaceous and sweat glands. A variety of cells can be found in the dermis, e.g., fibroblasts, macrophages, and mast cells. The dermis is divided into two layers, papillary dermis and reticular dermis. The cutaneous blood supply is arranged as two horizontal vascular networks, one at the dermal-subcutaneous junction and the other at the subpapillary level. From the subpapillary horizontal plexus capillary loops rise up into dermal papillae, usually one per papilla. The direction of blood flow in this network is from the arterioles to venules. Figure 2 shows the general layout of the vasculature.
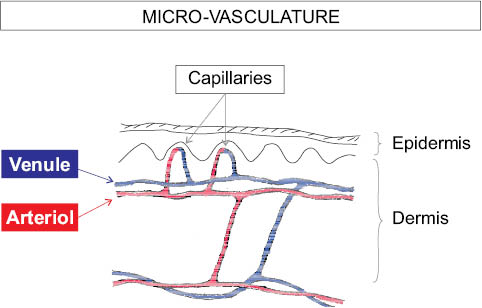
Figure 2: Microvasculature of the skin.
The main barrier to the skin is provided by stratum corneum. It protects skin against outside chemical insults and controls the trans-epidermal water loss (TEWL). The flattened cells of the SC, corneocytes, are continuously being renewed as a result of a process of epidermal maturation and terminal differentiation. The corneocytes are embedded in a matrix of intercellular lipids (brick and mortar model). They are also riveted together by intercellular protein structures called corneodesmosomes. There may also be ionic bonding between the corneocytes. Each corneocyte is a protein complex encapsulated within a protein shell called the cornified cell envelope. Within the corneocytes reside a number of water-soluble compounds collectively called natural moisturizing factor (NMF), which have the ability to bind water. Both the intercellular lamelar lipids and the natural moisturizing factor maintain the balance of water in a healthy stratum corneum. Lack of water can result in dry, brittle, cracking, and rough skin.
in most clinical studies, evaluations are performed combining visual grading of skin attributes as well as measurements by a variety of bio-instrumentations.
Following each laboratory’s “standard operating procedure” (SOP), the instruments are calibrated and tested on regular basis.
For some of the instrumental measurements, keeping the room temperature and humidity constant is critical—for example, measurement of stratum corneum hydration and trans-epidermal water loss. Generally, a temperature of 21° ± 1° Celsius (70° ± 1° Fahrenheit) and relative humidity of either 40% ± 5% or 50% ± 5% are required for some types of skin evaluations.
An environmental chamber is the best way to control the temperature and humidity precisely.
A variety of types and sizes of environmental chambers are available. We use a closed chamber with a single entry door and with thick wall insulation for controlling the temperature and humidity. A highly precise process control unit is used for reading the values of temperature and humidity from the sensors; it also controls the ambient condition by sending pulses of hot, cold and humid air, along with cycles of dehumidification.
The mixing of hot, cold, and humid air is done outside the chamber, in a subchamber above the ceiling. The ceiling has 56,800 micro-holes, 3-mm diameter each and distributed 12 mm apart. The mixed air from above continuously filters through these micro-holes down into the chamber to provide a uniform atmosphere inside, and holds the temperature and humidity constant. In this system there is no air draft or substantial air movement inside the chamber, which is very different from an air-conditioned room where the fans blow the air into the room.
The inner dimensions of the chamber are: width 301 cm, length 355 cm, and height 213 cm. Outside the chamber there are refrigeration and heating units as well as humidity and dehumidifying units. The humidifying system consists of water mist producer (vapor injection). The water supplied to this unit comes through a two-stage laboratory water filter. The dehumidifier system consists of a large freezer coil with condenser fins, placed just outside the wall, across a screened opening.
The temperature and humidity are continuously recorded on a chart recorder. The recommended time for acclimation of subjects is between 15 and 30 minutes, but could be longer for some experiments.
Proper level of stratum corneum (SC) hydration is essential for healthy skin. Lack of moisture leads to rough, dry skin and can cause a number of skin ailments.
Skin moisture can be measured in many ways but by far the most common method is to measure the electrical impedance of skin. Hydration changes in the stratum corneum influence the electrical capacitance and conductance of skin. Three of the most common instruments these days are: novameter, skicon, and corneometer. These are simple and easy to use instruments utilized by dermatologists to follow skin disease during treatment as well as by industry to test moisturizers and topical drugs. Figure 3 shows the three hydration probes, which are placed in contact with skin surface.
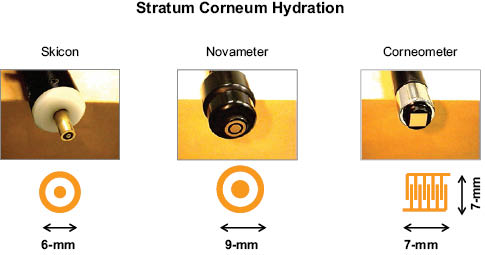
Figure 3: The hydration measuring probes of Skicon, Novameter, and Corneometer. The electrode dimensions are also shown.
The Skicon (Skicon-200, I.B.S. Co. Ltd. Japan) is a conductance-measuring device with concentric circular electrodes. It uses a fixed electrical frequency of 3.5 megahertz. The measurements are given in microsiemen (mS) units and have a range of 5 to 1999.
The Novameter (Model DPM9003, Nova Technologies, NH) is an impedance-measuring device with concentric circular electrodes separated by a gap of 1 mm. It uses varying electrical frequencies over a range up to 1 MHz, and the capacitance is derived from the signal-phase delay. The measurements are given in arbitrary units referred to as DPM units (DPM stands for Dermal Phase Meter). The range is 90 to 999.
Corneometer (model CM 825, Courage + Khazaka electronic GMBH, Germany) is a capacitance device, which consists of a pair of electrodes formed as an interdigital grid with small spacing between the elements. Readings are given in arbitrary units.
In a more sophisticated mode these instruments can be used to determine the ability of skin to be moisturized and its capacity to hold water. This is called the sorption-desorption test, in which a drop of water is applied to skin for a brief period and the dynamics of hydration is followed through a series of moisture measurements. Using this test we find the water-holding capacity, hygroscopicity, and desorption rate constant of skin in health and disease.
Another test is the moisture accumulation test (MAT), in which the skin is occluded with the measuring probe for a period of time and the increase in hydration is continuously recorded. Using these instruments, efficacy of moisturizers can be tested.
11.5.6 TRANS-EPIDERMAL WATER LOSS
Another important phenomenon in skin is the loss of water through epidermis, which essentially relates to the barrier function of skin. This is measured by an instrument that measures the water evaporation from a surface by detecting the partial pressure gradient of water vapors. Two pairs of sensors, measuring humidity and temperature, are arranged in a cylindrical well in such a way that one pair is farther than the other from the surface whose evaporation rate is to be measured. The schematics of this arrangement are shown in Figure 4. The reading displayed is called trans-epidermal water loss (TEWL) and is given in units of g/m2h. In general, skin irritation, barrier disruption, and sweat output increase the TEWL. Effects of various cosmetics, toiletries, and topical drugs can be studied with this instrument. Various dermatoses can also be followed.
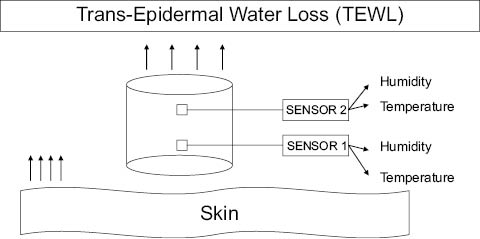
Figure 4: Measurement of Trans-Epidermal Water Loss using two pairs of humidity and temperature sensors.
Evaporimeter (Model EP2, Servomed, Sweden) measures TEWL in an area of 12-mm diameter circle. Measurements are done in an environmental chamber at a temperature of 70° ± 0.5°F and a relative humidity of 40 ± 5%. Readings are recorded between 45 and 60 seconds after placing the probe on the skin surface. The data can also be collected through an EP2 program. The TEWL is given in g/m2h.
The Dermalab TEWL instrument (Dermalab, Cortex Technology, Denmark) has a probe diameter of 11 mm. Measurements are done in an environmental chamber at a temperature of 70° ± 0.5°F and a relative humidity of 40 ± 5%. Readings are recorded at 60 seconds post-probe contact with the skin surface. The data can also be collected through a program. The TEWL is given in g/m2h.
11.5.7 SKIN BLOOD FLOW, COLOR, ERYTHEMA
The redness is caused by increased blood flow in the superficial capillaries in skin. It is important to measure erythema during treatment of skin diseases as well as during safety testing of topical products. It is also useful to test skin by provoking erythema reaction (irritation model) and then test the ability of a topical product in reducing erythema. Various instruments have been devised to measure erythema. Some are based on measuring the movement of erythrocytes and others are based on measuring the color or spectra of the light reflected off the skin. Some of the common instruments are: Laser Doppler flowmeter, colorimeter, and diffuse reflectance spectrophotometer. The inflammatory reactions of skin can be studied, e.g., due to a variety of stimuli, e.g., irritants, vasodilators, UV exposure, mechanical abrasion, topical application of products, and drugs.
The Laser Doppler Flowmeter works on the principle that light reflected off the moving erythrocytes experience a Doppler shift in frequency. The interaction of the frequency-shifted light waves with the nonshifted light waves produces a signal that is proportional to the blood flow in the capillaries. The laser Doppler imager (MoorLDI, Moor Instruments, Devon, UK), scans a laser beam on a predetermined rectangular area on the skin surface by a moving mirror that produces a raster pattern. The Doppler-shifted signal is recorded as an image of 250 × 250 pixel. The blood-flow values at each pixel are given in an arbitrary “Perfusion Unit (PU),” which is proportional to the product of “number of erythrocytes” and their “mean velocity” in a volume of tissue. Figure 5 shows the schematics of the Laser Doppler Imager. A Helium-Neon (He-Ne) Laser is used with a wavelength of 632.8 nm.
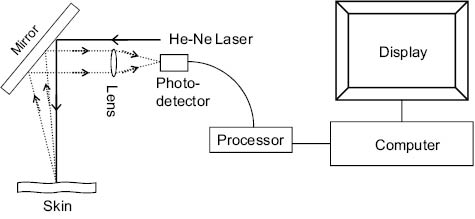
Figure 5: Schematics of the Laser Doppler Scanner.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







