Key Points
- ▪
Lymphedema severity may require different treatment approaches. The indications for referral to conservative therapy are different from those for referral for surgery.
- ▪
Knowing the extent to which the patient is engaged with therapy helps focus the treatment approach and plan.
- ▪
Although lymphedema is generally considered to be a ‘painless’ condition, a variety of symptoms, including pain, tenderness, heaviness, and firmness/tightness, can accompany this disorder and be quite distressing.
- ▪
Symptoms are important when setting goals for treatment, since what the patient considers a successful outcome may differ from what is considered successful for the therapist, physician, or healthcare system.
- ▪
The main objective of lymphedema management is volume reduction and maintenance. Although limb volume is not the sole outcome, identifying when a patient enters volume stabilization is crucial for decision-making regarding further long-term management.
- ▪
Evidence in the literature supports the reliability of multiple approaches for the assessment of the swollen limb. It is important to note that while multiple measurement modalities are valid and reliable, they are not interchangeable; the selected method must be done repeatedly over time to assess for change.
- ▪
It is the clinician’s role to provide the best diagnosis, treatment plan, and advice as to what will be the most appropriate management; taking into consideration of the stage and severity of lymphedema together with psychosocial status can help predict the amount of participation the patient (and/or caregiver) will be able to contribute to the process.
Introduction
Lymphedema is not a life-threatening condition in the majority of patients; however, it causes physical, functional, emotional, and social distress that can exceed the severity of the condition. The severity of lymphedema may require different treatment approaches. Assessment should begin with a thorough history and physical examination to establish a correct diagnosis and care plan. Each phase of the clinical evaluation must be purposeful to ensure that the patient does not go through unnecessary tests. Lymphedema is a chronic condition for which a cure has not yet been identified; nevertheless, when diagnosed early, intervention may reverse or reduce the condition to the pre-emergent state or minimize its debilitating effects. The indications for referral to conservative therapy are different from those for referral for surgery. This chapter will emphasize the phases that need to be addressed in the clinical evaluation.
Patient History and Assessment
The purposes of a thorough patient history are to identify risk factors for developing lymphedema, identify the cause(s) of swelling, and to explore whether there are contraindications or precautions for treatment.
Medical History
Risk factors for developing lymphedema for those who have no prior symptoms are outlined in Box 7.1 : Patients may have undergone procedures or treatments that put them at higher risk to develop lymphedema. The most investigated group is patients undergoing treatment for breast cancer; it had been shown that the extent of axillary lymph nodes dissection, axillary radiation, high body mass index (BMI), postoperative infection, and seroma are all associated with a higher risk of developing lymphedema. In a recent study, a small number of gene mutations/haplotypes that are associated with primary lymphedema were found in women after breast cancer treatment, suggesting genomic risk factors for developing lymphedema in this population. Breast cancer survivors are at lifetime risk of developing lymphedema and therefore need to undergo surveillance. Armer et al. reported that even women whose limb volumes were stable for six months after surgery prior to experiencing a 5% increase in volume, had a 94% chance of later developing lymphedema (defined as ≥10% limb volume increase). Another example is a study by Damstra et al. that found lymphatic dysfunction in patients’ affected lower extremities after one episode of an erysipelas attack, a dysfunction which was evident in the clinically nonaffected leg as well. These findings suggest that people who experience an erysipelas attack are at risk of developing lymphedema and should undergo surveillance and/or engage in risk-reduction activities. Considering the importance of understanding risk factors for development of lymphedema and the limitations in our current knowledge, rigorous research with well-defined outcomes, adequate patient sample sizes, and prospective surveillance is imperative.
Upper Limb/Trunk Lymphedema
Age
Family or personal history of lymphedema
Genetic predisposition to lymphedema
Pre-morbid conditions
Medications causing fluid retention/imbalance
Body mass index
Type of surgery and node dissection
Re-excision of tumor for clear margins
Breast radiation
Axillary radiation
Chemotherapy (e.g., taxane)
Postoperative trauma and infection(s)
Weight gain during/after treatment
Cording and seroma formation
Congenital predisposition
Insertion of pacemaker
Arteriovenous shunt for dialysis
Living in or visiting a lymphatic filariasis endemic area
Trauma in an ‘at-risk’ arm (venipuncture, blood pressure measurement, injection)
Lower Limb Lymphedema
Inguinal, suprafemoral, para-aortic, pelvic, and iliac lymph node dissection
Postoperative radiotherapy
Recurrent soft tissue infection at the same site
Surgery or trauma to the limb (e.g., total knee replacement)
Obesity
Varicose vein stripping and vein harvesting
Genetic predisposition/family history of chronic edema
Advanced cancer
Intrapelvic or intra-abdominal tumors that involve or directly compress lymphatic vessels
Poor nutritional status
Thrombophlebitis and chronic venous insufficiency, particularly post-thrombotic syndrome
Any unresolved asymmetrical edema
Chronic skin disorders and inflammation
Concurrent illnesses such as phlebitis, hyperthyroidosis, kidney or cardiac disease
Immobilization and prolonged limb dependency
Living in or visiting a lymphatic filariasis endemic area
Risk factors for lymphedema of the upper and lower limbs based on published literature.:
- 1.
Bevilacqua JL, Kattan MW, Changhong Y, et al. Nomograms for predicting the risk of arm lymphedema after axillary dissection in breast cancer. Ann Surg Oncol 2012 Aug;19(8):2580-9. PubMed PMID: 22395997. Epub 2012/03/08. eng.
- 2.
Miaskowski C, Dodd M, Paul SM, West C, et al. Lymphatic and angiogenic candidate genes predict the development of secondary lymphedema following breast cancer surgery. PloS one. 2013;8(4):e60164. PubMed PMID: 23613720. Pubmed Central PMCID: 3629060.
- 3.
Stout NL, Binkley JM, Schmitz KH, et al. A prospective surveillance model for rehabilitation for women with breast cancer. Cancer 2012 Apr 15;118(8 Suppl):2191-200. PubMed PMID: 22488693.
- 4.
Green JM, Paladugu S, Shuyu X, et al. Using temporal mining to examine the development of lymphedema in breast cancer survivors. Nurs Res 2013 Mar-Apr;62(2):122-9. PubMed PMID: 23458909. Epub 2013/03/06. eng.
- 5.
Damstra RJ, van Steensel MA, Boomsma JH, et al. Erysipelas as a sign of subclinical primary lymphoedema: a prospective quantitative scintigraphic study of 40 patients with unilateral erysipelas of the leg. Brit J Dermatol 2008 Jun;158(6):1210-5. PubMed PMID: 18363756.
- 6.
Cemal Yeliz AP, Babak J Mehrara. Preventative Measures for Lymphedema: Separating Fact from Fiction. J Am Coll Surg 2011;213(4):543-51.
- 7.
Best practice for the management of lymphedema. International consensus document. London: MEP; 2006.
Known Etiology of Lymphedema
Primary lymphedema is due to dysplasia of the lymphatic system. Primary lymphedema can be clinically classified as ‘congenital lymphedema’ which can manifest as swelling from birth to two years of age; ‘lymphedema praecox’, from two to 35 years of age; or ‘lymphedema tarda’, onset after 35 years of age. In most cases, a malformation of the lymphatic system will be evident in imaging (e.g., aplasia, hyperplasia, or hypoplasia). Currently ten gene mutations have been identified that are associated with lymphedema. These include vascular endothelial growth factor receptor 3 ( VEGFR3 ) that is associated with some familial Milroy lymphedema, first described in 1998, to more recently, gap junction protein, alpha 1 (GJA1) in oculodentaldigital-lymphedema, and vascular endothelial growth factor C ( VEGFC ) in Milroy-like lymphedema, both described in 2013 (for more details see reference ). Others still are yet to be identified, as there are classified lymphedema-associated syndromes without identified genetic mutations.
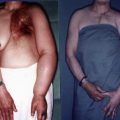
Secondary lymphedema is associated with an extrinsic event (e.g., cancer, radiation, vascular disorders, trauma, skin infections, operations). The most researched etiology for lymphedema is that secondary to breast cancer. This may be due to the large numbers in which lymphedema occurs and the years of survivorship possible with modern cancer treatment, as well as the high visibility of the swollen upper extremity. Lymphedema secondary to breast cancer can manifest itself in swelling of the whole upper quadrant of the truncal regions (front and back of the chest wall and arm); however, usually swelling (and sensation changes) will start in a specific region and in time will progress to other territories. Stanton et al. demonstrated in their study that the mechanism is not similar to a stopcock, rather the lymphatic change represents a regional swelling. Therefore, lymphedema can start at the forearm or the hand and proceed proximally or at the upper extremity proximally and proceed distally. Another cause of secondary lymphedema is venous insufficiency in which the venous hypertension exceeds the lymphatic transport capacity leading to chronic edema, complicated frequently by chronic ulcers.
Comorbidities
This involves other conditions that may cause swelling or exacerbate lymphedema (see Box 7.2 : Differential Diagnosis) or that may be considered contraindications for lymphedema treatment ( Box 7.2 ). An example is chronic heart failure in which both legs may be edematous. This type of swelling should be treated with the appropriate cardiac care. However, if swelling persists after elevation and medication for the cardiac condition, lymphedema compression bandaging (LCB) can shift fluid centrally. A cardiologist should be consulted as treatment begins and continues.
Unilateral Limb Swelling
Acute deep vein thrombosis (DVT) (CI for PIC)
Post-thrombotic syndrome
Arthritis
Baker’s cyst
Presence/recurrence of malignancy
Symmetrical Swelling
Congestive heart failure (CI for PIC, LCB)
Renal dysfunction (CI for PIC, MLD)
Hepatic dysfunction
Hypoproteinemia
Hypothyroidism/myxedema
Lipoedema
Idiopathic sodium retention
Severe arterial insufficiency (ABPI <0.5), (CI for PIC, MLLB)
Severe peripheral neuropathy (CI for PIC, MLLB)
Pulmonary embolism (CI for PIC)
Acute inflammations of the skin, e.g. cellulitis/erysipelas (CI for PIC, MLD)
Pulmonary edema (CI for PIC)
Active metastatic disease affecting edematous region (CI for PIC)
Superior vena cava obstruction (CI for MLD)
Tuberculosis and/or malaria (CI for MLD)
Unstable hypertension (CI for MLD)
Hepatic cirrhosis with abdominal fluid (ascites) (CI for MLD)
LCB: Lymphedema compression bandaging
MLD: Manual lymphedema drainage
PIC: Pneumatic intermittent compression
ABPI: Ankle-brachial pressure index
Medications
Drugs that have a side effect of swelling ( Box 7.3 ) can either cause or exacerbate lymphedema. Swelling will not always appear immediately after taking the drugs; therefore, establishing causality is difficult. However, when there are other drugs available that do not cause swelling, these patients may benefit from these alternatives. When there is no possibility of replacing the drug and the edema is manageable (for example, when swelling disappears with elevation), compression stockings can be provided for lymphedema care.
Calcium channel blockers (amlodipine, felodipine, nifedipine, diltiazem)
Corticosteroids (e.g., prednisolone and dexamethasone, fludrocortisone)
Nonsteroidal anti-inflammatories (e.g., diclofenac, Ibuprofen, naproxen, celecoxib)
Alfa blockers (e.g., doxazosin)
Sex hormones and related compounds (estrogen (hormone-replacement therapy), anastrozole, tamoxifen, megestrol)
Antipsychotics (e.g., risperidone, fluphenazine, olanzapine)
Anticonvulsants (e.g., pregabalin, gabapentin)
Antidepressants (e.g., trazodone, mirtazapine, paroxetine)
Antidiabetics (e.g., rosiglitazone, pioglitazone)
Anti-parkinsonians (e.g., amantadine, cabergoline, ropinirole)
Bisphosphonates (e.g., for cancer treatment: zoledronic acid, risedronate, tiludronate)
Cytotoxic chemotherapy (e.g., docetaxel)
Sirolimus—decreases the action of the immune system
Potassium channel activator—diazoxide for hypoglycemia
Minoxidil ( Rogaine)—alopecia androgenetica in both men and women
Proton pump inhibitors (e.g., esomeprazole, omeprazole, lansoprazole, pantoprazole)
Other drugs (anagrelide, atorvastatin, cilostazol, ciprofloxacin, etretinate, glatirimer acetate, Isosorbide dinitrate, Itroconazole, metoclopramide, nicotinic acid, orlistat, pentoxifylline, tacrolimus, voriconazole)
Blood tests:
- ▪
complete blood count
- ▪
urea and electrolytes
- ▪
thyroid function tests
- ▪
liver function tests
- ▪
plasma total protein and albumin
- ▪
fasting glucose
- ▪
erythrocyte sedimentation rate/C-reactive protein
- ▪
β-natriuretic peptide
- ▪
Urine dipstick testing, including observation for chyluria
Ultrasound
Chest X-ray
Social Situation and Level of Support
Armer et al. published a model that showed that social support and coping style are protective mechanisms for outcomes of breast cancer-related lymphedema, such as quality of life and functional health status. In a clinical setting, a patient who has support from a family member may be able to adhere to the treatment regimen more readily than the patient who is coping alone.
Medical Assessment and Diagnosis
Screening Tests ( Box 7.4 )
The purpose of screening tests is to assist the physician to determine the etiology of the edema when clinical manifestation is not sufficient. For example, redness of the skin that accompanies swelling can be caused by erysipelas infection. However, lymphangiosis carcinomatosa (an inflammation of the lymph vessels which can be associated with cancer) can manifest in the same way. Laboratory assessment for infection may demonstrate a pronounced leukocytosis, elevated C-reactive protein (CRP), and the blood cultures which are positive for group A streptococci (indicating erysipelas) or Staphylococcus aureus.
Lymphatic System Functional Assessment
Lymphoscintigraphy is the current ‘gold standard’ imaging test for functional assessment that uses a tracer molecule linked to technetium-99m that is injected into the dermis in the foot and/or hand. This imaging test can evaluate dynamic response (reduced flow), areas of blockage, and dermal backflow. It is indispensable in cases of complicated lymphedemas (i.e., chylous and nonchylous reflux, lymphangiodysplasia, etc.). Hwang et al. found that a baseline lymphoscintigraphy can predict a good response to complex decongestive therapy (CDT) in patients whose dominant lymphatics were imaged. For those patients who had visualized collateral lymphatic vessels without a main vessel, the response to CDT was poor. This important information can motivate people with a potential for a good CDT response to be actively invested in their treatment. This imaging helps to define the lymphatic problem and the possible response to treatment or direction of treatment. These images can offer confirmation of their previously undiagnosed condition and can help focus their efforts on treatment with their clinicians.
Fluorescence lymphography uses indocyanine green (ICG) and infrared fluorescence for imaging the lymphatic system. Although found to correlate with lymphoscintigraphy for superficial imaging, the system cannot detect lymph vessels and structures deeper than 2 cm. Therefore, ICG imaging lacks the ability to visualize and provide a complete image of the lymphatics. Although improved results and analyses continue to be reported; the lack of deeper lymphatic system imaging precludes its use for patients with more than superficial alterations in their lymphatic system.
Ankle-Brachial Pressure Index (ABPI)
As compression influences microcirculation, as well as venous and arterial flow, caution should be applied when vascular disturbances are detected. Compression is prohibited in the presence of critical ischemia (ABPI <0.5); however, in patients with low ABPI (0.5–0.8), compression may be applied for reduction of edema.
Color Doppler Ultrasound
This modality is used to assess deep vein thrombosis (DVT) and venous abnormalities. The existence of a DVT does not prevent referral for compression therapy. LCB is known to be safe in the acute phase of DVT with no long-term effect on the development of post-thrombotic syndrome, valve incompetency, or thrombus regression.
Computed Tomography (CT) and Magnetic Resonance Imaging (MRI)
These imaging studies can be used to detect skin thickening and subcutaneous swelling. Noninvasive MRI can assess lymph flow in vivo without administration of exogenous contrast agent. Several investigators have demonstrated high spatial-resolution imaging with the use of gadolinium contrast agents. However, the two-dimensional MRI gives limited information as to dilated lymphatics. A three-dimensional imaging study (vs. lymphoscintigraphy) was found to be more informative. In this report, lymphatic anatomy and obstruction were identified, as well as the effect of obstruction on local structures and tissue composition.
Lymphedema Characteristics
These data are important for planning management according to patient goals, prognosis, expectations, and ultimately understanding how to invest energy and effort.
Type (Primary versus Secondary)
Knowing the lymphedema type will not change the decision as to what conservative management will be offered to the patient. However, if surgical interventions are considered, lymphedema type is important information. A nonsurgical example is a patient who has primary lymphedema of one limb and may be at risk to develop lymphedema in other limbs, especially if there are known anatomical defects; therefore, a holistic strategy that will target other areas, such as exercise, or even compression for prevention, can be addressed. Patients with familial primary lymphedema may also choose to undergo genetic evaluation for known genes if they have siblings who may be at risk or if they wish to have children in the future.
Location
Swelling can manifest in the extremities where compression bandaging and garments are more easily applied. However, areas such as face, neck, genital area, and chest (midline lymphedema) are more challenging for application of compression. Swelling of these areas may benefit from the addition of other treatment modalities, such as aqua lymphatic therapy and kiniseo taping, which have not yet been fully studied to provide high-level evidence.
Symptoms
Although lymphedema is generally considered to be a ‘painless’ condition, a variety of symptoms, including pain, tenderness, heaviness, and firmness/tightness, can accompany this disorder and be quite distressing. Symptoms such as heaviness and swelling have been found to correlate with the physical findings of lymphedema. Symptoms are important when setting goals for treatment, since what the patient will consider a successful outcome (e.g., reduced symptoms), may differ from what is considered successful for the therapist (e.g., reduction of edema) or the health system.
What Makes the Edema Better?
One of the main issues in deciding which lymphedema classification to choose is the response to elevation. For example, stage I lymphedema is when edema subsides after elevation. Knowing what makes lymphedema better helps in planning treatment and what advice to give to patients.
Presence of Wounds
Chronic ulcers of various etiologies can occur when swelling is involved and can benefit from compression bandaging. However, proper wound care should be administered and all the information regarding chronicity, depth, size, and treatment should be obtained in order for a good collaboration between healthcare providers and the lymphedema therapist.
Self-Management
Adherence to compression garment or bandaging has proven to be the most effective means in maintaining the results of intensive therapy. Knowing whether the patient with lymphedema is engaged with therapy helps focus the treatment approach and plan. In addition, close adherence to management strategies using exercises, Manual lymphedema drainage (MLD), and bandaging will aid predicting likelihood of success with certain other treatment protocols, including surgery.
Assessment Techniques
Observation
Every assessment begins with clinical observation. A step-wise approach will help to guide the clinician to perform a thorough assessment. Many important disease entity characteristics can be determined through the physical exam.
Functional Status
Functional status can be observed from the moment a patient walks into the clinic.
Examples of functional observation of the lower extremities include the following:
- ▪
Does the patient have a limp?
- ▪
Does he or she use an ambulation aid?
- ▪
Can he or she bend a leg to a half-tailor position when taking shoes or socks off (may direct to restricted range of motion in the hip joint)?
- ▪
Can the patient make a forward stride (the extended leg can be restricted by a scar in the groin area)?
For upper extremities:
- ▪
Is the patient independent in donning/doffing a shirt or bra?
- ▪
Is there restricted shoulder motion/weakness?
Patients with functional problems should be referred to physical therapy or occupational therapy services for evaluation and treatment.
Swelling
Areas of indentation (bra, underwear, watch, jewelry, socks), asymmetry between limbs, or areas of swelling may be the first signs of swelling. Swollen segments (e.g., hand, upper arm, genitals) can lead to more extensive swelling, or may be the only area of swelling. Nevertheless, swelling is an indication for treatment.
Skin Condition
Box 7.5 summarizes different skin conditions, including wounds, that can accompany swelling. For example, dry skin can put a patient at risk for cellulitis or fungal infection. Infections need to be treated prior to the initiation of lymphedema therapy. Stemmer’s sign is positive when the skin of a digit is fibrotic and cannot be lifted by the examiner’s fingers ( Figure 7.1 ). The compliance of the skin determines if the test is positive or negative; whereas the ability to grasp the skin and lift it is a negative sign for lymphedema. The absence of Stemmer’s sign does not rule out the diagnosis of lymphedema.