Developmental Biology
An understanding of head and neck embryology is helpful in the appreciation of the wide spectrum of the cleft lip and palate phenotype. The cranial portion of the human embryo develops early, with the three germ layers (ectoderm, mesoderm, and endoderm) forming in the beginning to middle of the third week of gestation. The ectoderm layer gives rise to the cutaneous and neural systems, with differentiation starting at 20 days. The interaction between ectoderm-derived components at the crest of the neural fold gives rise to a unique cell population of neural crest cells (NCCs). NCCs have the unique ability to remain pluripotent despite their single germ layer origin. NCCs migrate along cleavage planes between germ layers and within the mesoderm to differentiate at their final destination into connective, muscle, nervous, or endocrine tissue, as well as pigment cells.
NCCs that migrate ventro-caudal from the crest come into contact with the pharyngeal endoderm and mesoderm core that surrounds the six aortic arches. This results in a series of mesenchymal swellings termed branchial arches in the fourth week. The six paired branchial arches decrease in size from cranial to caudal. Although the first and largest arch, the mandibular arch, is primarily responsible for development of the anatomy that includes the lip and palate, the fourth arch is responsible for the pharyngeal constrictor, the levator veli palatini, and the palatoglossus muscles, which play a role in the problems and treatments associated with cleft palate. Each branchial arch gives rise to a nerve along with the associated muscles. This muscle-nerve relationship is maintained regardless of the functional interaction of the differentiated structures. Although the tensor veli palatini and levator veli palatini work in close coordination in the mature normal palate and are pathologically tethered through the aponeurosis of the tensor tendon in patients with cleft palate, they retain their distinct innervation based on their embryologic origin. The levator veli palatini, as a fourth arch derived muscle, is innervated by the fourth arch derived superior laryngeal branch of the vagus (cranial nerve X). The fourth arch derived palatoglossus and pharyngeal constrictors have similar innervation. The tensor veli palatini alone, as a derivative of the first arch, is innervated by the trigeminal nerve (cranial nerve V).
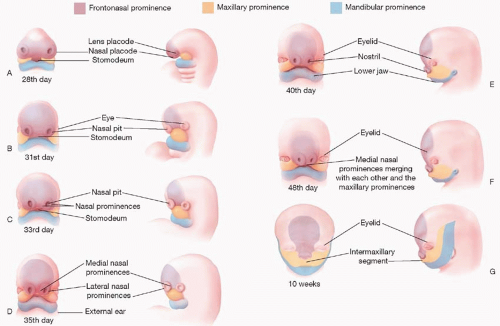
The first branchial arch and the mesenchyme ventral to the developing forebrain are responsible for the three named prominences that give rise to the face, mouth, neck, larynx, pharynx, and nasal cavities (
Figure 19.1). The first branchial arch contributes the paired
maxillary and
mandibular prominences, which fuse to form the lateral and caudal components of the primitive stomodeum or mouth. A central process formed by the proliferation of mesenchyme ventral to the forebrain creates the
frontonasal prominence (FNP), which forms the cranial portion of the stomodeum. It is important to note that the FNP and its derivatives are not formed by branchial arches, but rather originate from distinct mesenchyme ventral to the branchial arches. These five facial prominences (two paired and one unpaired) are separated by external grooves, but the mesenchyme of all five is continuous, such that unobstructed migration of mesenchymal cells can occur around the stomodeum. Coordinated fusion and communication between these five prominences are essential for normal lip and palate development.
Development of the human face occurs between the 4th and 10th weeks (
Figure 19.1B-H). The nasal placodes develop as bilateral thickenings on the surface ectoderm of the infero-lateral aspect of the FNP by the end of the fourth week (
Figure 19.1C). As the placodes elevate, medial and lateral nasal prominences develop around the depressed central nasal pit. Medial migration of the maxillary prominence from the first arch effects medial migration of the nasal prominences, such that when they fuse together, the stomodeum is no longer in continuity with the nasal pit, creating the nasal-oral separation (
Figure 19.1G).
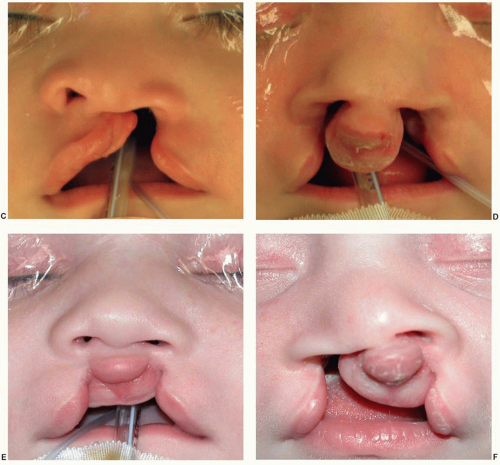
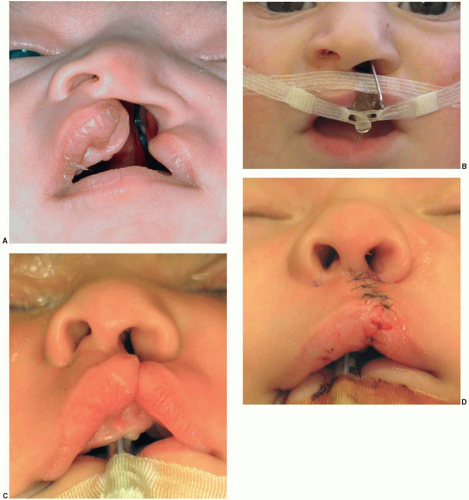
The medial nasal prominences form the philtrum and Cupid’s bow region of the upper lip, the nasal tip and septum, and the premaxilla back to the incisive foramen. The lateral nasal prominences form the nasal alae. The maxillary prominences form the lateral lip elements that normally fuse with the philtrum derived from the medial nasal prominence. A failure of fusion of a lateral lip element (maxillary prominence) with the philtrum (medial nasal prominence from the FNP) results in a unilateral cleft lip. If both maxillary prominences fail to fuse, a bilateral cleft lip will result. With a failure of fusion to the maxillary prominence, the growth of the medial placode elements (prolabium, premaxilla, and septum) is unbalanced, resulting in the central protrusion seen in a cleft patient.
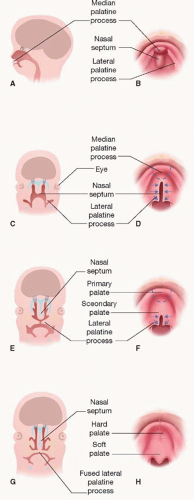
The formation of the palate is also a result of interaction between the FNP and maxillary prominences. The two medial nasal prominences of the FNP merge to form the median palatine process, which develops the primary palate, whereas the
lateral palatine processes derived from the maxillary prominences form the secondary palate (
Figure 19.2). During the eighth week, the lateral palatine processes change from their initial vertical orientation to horizontal, within a period of hours. The developing mandible protrudes in synchrony to allow the tongue to descend and leave room for palate fusion. Fusion occurs in both the axial and sagittal planes, with the median palatine process and two lateral palatine processes fusing to form the palate, and the nasal septum descending from the FNP to join the fusion and separate the two nasal cavities (
Figure 19.2C-F). Fusion involves focal degeneration of the leading epithelial edges in a process felt to represent “programmed cell death.” Once fused, the mesenchyme of the primary palate and anterior secondary palate ossify into the hard palate, whereas the posterior secondary palate forms muscle to create the dynamic soft palate.
When there is normal fusion between the FNP and maxillary prominences creating a normal lip and alveolus, but there is lack of fusion between the lateral palatine processes of the opposing maxillary prominences, an isolated cleft of the secondary palate occurs. If, however, the maxillary prominences fuse appropriately, creating a normal secondary palate, but the FNP and maxillary prominences do not fuse, then a cleft lip and cleft of the primary palate will occur. The variety of fusion patterns between these two pathologic scenarios results in the plethora of cleft lip and palate combinations described later in the chapter.
Epidemiology and Etiopathogenesis
Among the cleft lip and palate population, the most common diagnosis is cleft lip and palate (46%), followed by isolated cleft palate (33%) and isolated cleft lip (21%). The majority of bilateral cleft lips (86%) and unilateral cleft lips (68%) are associated with a cleft palate. Unilateral clefts are nine times as common as bilateral clefts and occur twice as frequently on the left side than on the right. Males are predominant in the cleft lip and palate population, whereas isolated cleft palate occurs more commonly in females. In the Caucasian population, cleft lip with or without cleft palate occurs in approximately 1 in 1,000 live births. These entities are twice as common in the Asian population, and approximately half as common in African Americans. This racial heterogeneity is not observed for isolated cleft palate, which has an overall incidence of 0.5 per 1,000 live births.
Both environmental teratogens and genetic factors are implicated in the genesis of cleft lip and palate. Intrauterine exposure to the anticonvulsant phenytoin is associated with a 10-fold increase in the incidence of cleft lip. Maternal smoking during pregnancy doubles the incidence of cleft lip. Other teratogens, such as alcohol, anticonvulsants, and retinoic acid, are associated with malformation patterns that include cleft lip and palate, but have not been directly related to isolated clefts.
Genetic abnormalities can result in syndromes that include clefts of the primary or secondary palates among the developmental fields affected. More than 40% of isolated cleft palates are part of malformation syndromes, compared with less than 15% of cleft lip and palate cases. The most common syndrome associated with cleft lip and palate is van der Woude syndrome with or without lower lip pits or blind sinuses. Microdeletions of chromosome 22q resulting in velo-cardiofacial, DiGeorge, or conotruncal anomaly syndromes are the most common diagnoses associated with isolated cleft
palate. Although there is a recognized genetic component to nonsyndromic cleft lip and/or palate, it appears to be multifactorial. Among other recent studies, a meta-analysis of 13 genome scans by Marazita et al.
1 revealed multiple cleft lip/palate genes on 16 chromosomal regions.
Parents of a child with a nonsyndromic cleft, or a family history of clefting, always ask about the risk of clefts in subsequent pregnancies. The risk depends on whether the proband has a cleft lip alone (CL), cleft lip with cleft palate (CLP), or a cleft palate alone (CP). If the family has one affected child or parent with CLP, the risk of the child of the next pregnancy having CLP is 4%. If two previous children have CLP, the risk increases to 9%, and if one parent and one child were previously affected, the risk to children of subsequent pregnancies is 17%. For families with a child having CP, the risk of CP to children of subsequent pregnancies is 2%, 6% if one parent has CP, and 15% if one parent and one previous child have CP.