Cicatricial Pemphigoid: Introduction
|
Epidemiology
Cicatricial pemphigoid has been estimated to occur in approximately 1 person per million annually; females are affected 1.5–2.0 times as often as males.7–9 Cicatricial pemphigoid has a mean age of onset of the early to middle 60s.9 Although there is no known racial or geographic predilection, the HLADQB1*0301 allele has been shown to be significantly increased in frequency in patients with oral, ocular, and generalized bullous pemphigoid; amino acid residues at positions 57 and 71 to 77 of the DQB1 protein may represent a disease susceptibility marker.1,10–14
Etiology and Pathogenesis
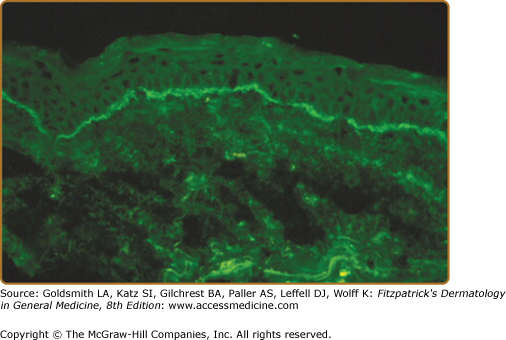
Autoantibodies directed against autoantigens in epidermal basement membrane are held responsible for the pathogenesis of cicatricial pemphigoid (Fig. 57-1).15 A variety of different autoantigens are recognized by circulating autoantibodies from these patients.1,16–31 These and other findings have led to the idea that cicatricial pemphigoid is not a single nosologic entity but rather a disease phenotype. Autoantigens recognized by immunoglobulin G (IgG) autoantibodies from patients with cicatricial pemphigoid are summarized in Table 57-1. While autoantibodies directed against some of these autoantigens have been shown to be pathogenic in vivo (Table 57-1), it is conceivable that other mechanisms may contribute to the pathogenesis of cicatricial pemphigoid. For example, recent studies have demonstrated high stromal expression of IL-13 in CD3+ T cells from patients with ocular cicatricial pemphigoid and that these cells may contribute both profibrotic and proinflammatory stimuli to conjunctival fibroblasts.39
Autoantigen | MW (kDa) | Location SSS/Ultra | Passive Transfer Studies |
|---|---|---|---|
BPAG1 | 230 | Epid/HD | |
BPAG2 | 180 | Epid/HD–af | IgG vs. the NC16A domain of BPAG2 creates subepid blisters in newborn mice that resemble those seen in patients with BP.32,33 |
Integrin β4 | ∼205 | Epid/HD–af | |
Integrin α6 | ∼120 | Epid/HD–af | |
Laminin 332 | 400–440 | Derm/LL–LD interface | Exp IgG (intact IgG and Fab fragments alone) vs. laminin 332 create subepid blisters in newborn and adult mice that resemble those seen in patients with AECP.34 |
Patient IgG creates subepid blisters in human skin grafts on immunodeficient adult mice that resemble those seen in patients with AECP.35 | |||
Type VII collagen | 290 | Derm/AF | Exp and patient IgG vs. the NC1 domain of type VII collagen create subepid blisters in adult mice that resemble those seen in patients with EBA.36–38 |
Bullous pemphigoid antigen 2 (BPAG2) appears to represent a major cicatricial pemphigoid autoantigen; other autoantigens of particular interest include laminin 332, integrin subunit β4, integrin subunit α6, type VII collagen, and bullous pemphigoid antigen 1. Other patients with cicatricial pemphigoid have IgA antibasement membrane autoantibodies (alone or in conjunction with IgG antibasement membrane autoantibodies); the best characterized IgA autoantigen linked to the cicatricial pemphigoid phenotype is bullous pemphigoid antigen 2.1,40–42
Clinical Findings
Patients typically describe the onset of painful, erosive, and/or blistering lesions on one or more mucosal surfaces. A few skin lesions on the upper body are also sometimes noted. Associated symptoms are site specific as detailed later.
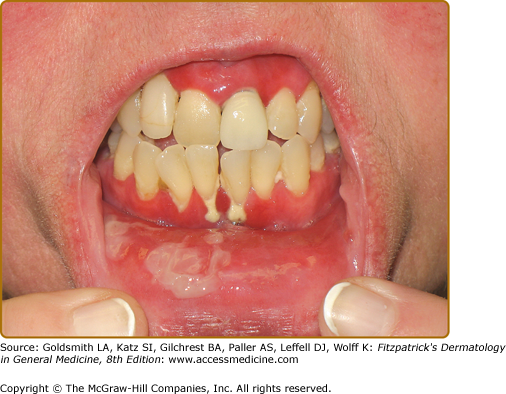
The mouth is the most frequent site of involvement in patients with cicatricial pemphigoid; it is often the first (and only) site affected. Lesions often involve the gingiva, buccal mucosa, and palate (Fig. 57-2); other sites such as the alveolar ridge, tongue, and lips are also susceptible.9,43 A frequent oral manifestation is desquamative gingivitis. Other lesions may present as tense blisters that rupture easily or as mucosal erosions that form as a consequence of epithelial fragility. Lesions in the mouth may result in a delicate white pattern of reticulated scarring. In severe disease, adhesions may develop between the buccal mucosa and the alveolar process, around the uvula and tonsillar fossae, and between the tongue and the floor of the mouth. Gingival involvement can result in tissue loss and dental complications (e.g., caries, periodontal ligament damage, and loss of bone mass and teeth).
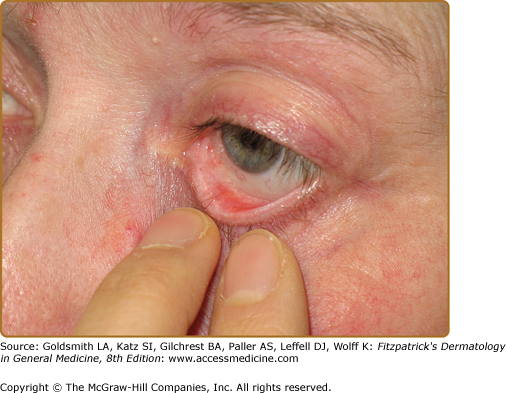
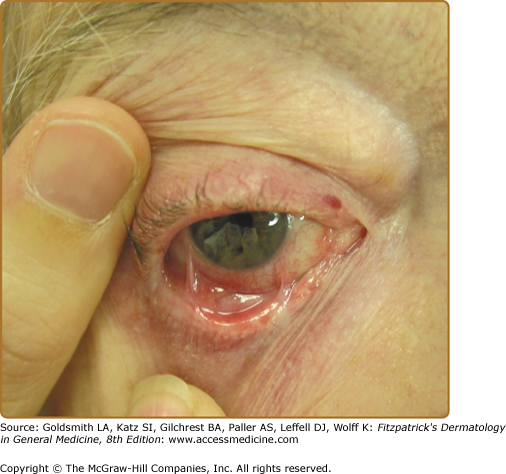
Ocular involvement in patients with cicatricial pemphigoid is common and may become sight threatening (Figs. 57-3 and 57-4). 44,45 Ocular lesions typically manifest as conjunctivitis that progresses insidiously to scarring. Early ocular disease can be quite subtle and nonspecific. Although disease is usually bilateral, it often begins unilaterally and progresses to both eyes within several years. Patients may complain of burning, dryness, or a foreign-body sensation in one or both eyes; frank blisters on conjunctival surfaces are rarely seen. Early disease is best appreciated by slit-lamp examination. Because disease may be localized to the upper tarsal conjunctiva, it may escape detection without eversion of the eyelids. Chronic ocular involvement can result in scarring characterized by shortened fornices, symblepharons (i.e., fibrous tracts between bulbar and palpebral conjunctival surfaces), and, in severe disease, ankyloblepharons (i.e., fibrous tracts fusing the superior and inferior palpebral conjunctivae with obliteration of the conjunctival sac). Conjunctival scarring also can cause entropion and trichiasis (i.e., in-turning of the eyelashes) that result in corneal irritation, superficial punctate keratinopathy, corneal neovascularization, corneal ulceration, and/or blindness. Additional ocular complications include scarring of the lacrimal ducts, decreased tear secretion, and loss of mucosal goblet cells leading to decreased tear mucus content and unstable tear films. It is very important for patients with suspected ocular involvement to be examined by an ophthalmologist, because early disease may be subtle, is only identified by slit-lamp examination, and can result in severe complications. Cicatricial pemphigoid may be limited to the eyes.
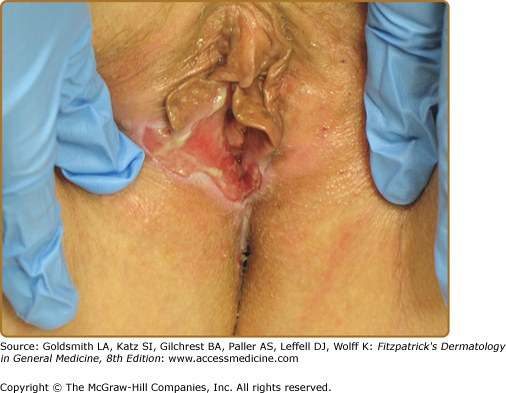
Other sites that may be affected by cicatricial pemphigoid include the nasopharyngeal, laryngeal, esophageal, and anogenital regions. Nasopharyngeal lesions can result in discharge, epistaxis, excessive crust formation, impaired airflow, chronic sinusitis, scarring, and tissue loss. Laryngeal involvement may present as hoarseness, sore throat, or loss of phonation. Chronic laryngeal erosions, edema, and scarring may result in supraglottic stenosis and airway compromise that eventually necessitates tracheostomy.46 Esophageal involvement may result in stricture formation, dysphagia, odynophagia, weight loss, and/or aspiration. Moreover, it has been suggested that esophageal dysfunction and gastroesophageal reflux may elicit or exacerbate laryngeal disease and/or bronchospasm in such patients. Although involvement of the genital and/or rectal mucosae in patients with cicatricial pemphigoid is rare, it can be a source of substantial pain and morbidity (Fig. 57-5). Rare cases of urethral stricture, vaginal stenosis, and anal narrowing have developed as a consequence of this disease.
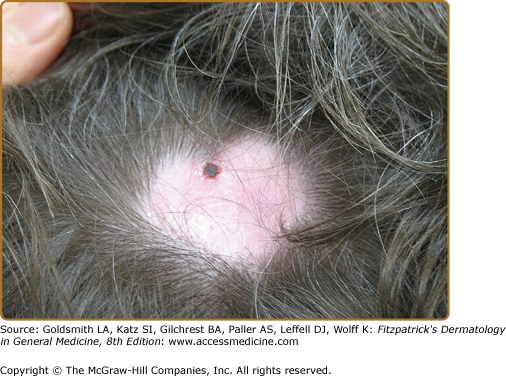
The skin is involved in 25%–35% of patients with cicatricial pemphigoid. The most frequently affected areas are the scalp, head, neck, and upper trunk (Fig. 57-6). Lesions typically consist of small vesicles or bullae situated on erythematous and/or urticarial bases. Lesions rupture easily and are often seen as small, crusted papules or plaques. The extent and number of cutaneous lesions are generally small; lesions sometimes recur in the same areas.
A cohort of 35 patients with antiepiligrin cicatricial pemphigoid (also called antilaminin 332 cicatricial pemphigoid) was shown to have an increased relative risk for cancer.47,48 Ten patients in this cohort had solitary solid cancers (three lung, three gastric, two colon, two endometrial); eight patients developed cancer after the onset of cicatricial pemphigoid (six within a year, seven within 14 months). The time between blister onset and cancer diagnosis was approximately 14 months in nine of the ten patients. Eight patients in this cohort died as a consequence of their cancer. All deaths occurred within 21 months. This form of cicatricial pemphigoid appears to have a relative risk for malignancy that approximates that for adults with dermatomyositis; as is true for the latter, the risk for cancer appears to be particularly high in the first year of disease. Other patients with this form of cicatricial pemphigoid and cancer have been described more recently.49–57 Interestingly, recent studies have suggested that the relative risk for cancer among patients with ocular or oral cicatricial pemphigoid and autoantibodies versus integrin subunit β4 or integrin subunit α6, respectively, may be reduced.58,59
In 1957, Brunsting and Perry described seven patients with locally recurrent and scarring subepidermal blistering lesions of the head or neck that for many years was thought to be a form of cicatricial pemphigoid.60 Although these patients are typically elderly and demonstrate deposits of immunoreactants in epidermal basement membranes like other patients with cicatricial pemphigoid, Brunsting–Perry pemphigoid predominates in men and lacks mucous membrane involvement. More recently, patients with the same clinical, histologic, and immunopathologic features have been reported to have autoantibodies directed against type VII collagen (or rarely to bullous pemphigoid antigens).3,61 Identification of similar patients with blister planes beneath the lamina densa further suggests that individuals with this phenotype usually have localized forms of epidermolysis bullosa acquisita.
Laboratory Tests
Although the findings of light microscopy studies of lesional skin or mucosa from patients with cicatricial pemphigoid often are nonspecific, they characteristically show a subepidermal blister and a dermal leukocytic infiltrate composed of lymphocytes and histiocytes as well as variable numbers of neutrophils and eosinophils.2,3,62,63 Plasma cells often are seen in mucosal lesions, whereas eosinophils and neutrophils are seen most commonly in skin lesions. Biopsy specimens of older lesions may be relatively “cell poor” and show features that correlate with the noninflammatory character of such sites clinically. Light microscopy studies of older lesions often show fibroblast proliferation and lamellar fibrosis (i.e., fibrosis characterized by collagen bundles ordered parallel to the surface epithelium).
Ultrastructural studies of lesional skin or mucosa from patients with cicatricial pemphigoid show that blisters typically develop within the lamina lucida and eventuate in partial or complete destruction of the basal lamina in older lesions.62–66 A generally held impression is that blisters form below those of bullous pemphigoid, because scarring is more common in patients with this disease. Reports of patients with blisters in the sublamina densa region are thought to represent mucosa-predominant forms of epidermolysis bullosa acquisita.
Direct immunofluorescence microscopy of normal-appearing perilesional tissue from patients with cicatricial pemphigoid shows continuous deposits of immunoreactants in epithelial basement membranes.5,66 The most commonly detected immunoreactants are IgG and C3 (see Fig. 57-1); the predominant subclass of these autoantibodies is IgG4.67 IgA, IgM, and/or fibrin are found in some patients.68 One study of skin and mucosal samples from ten patients found immunoreactants more commonly in perilesional mucosal biopsy specimens, which suggests that mucous membranes are the preferred biopsy site for direct immunofluorescence microscopy studies.66 Splitting tissue samples with 1 M NaCl increases the sensitivity of direct immunofluorescence microscopy and facilitates identification of immunoreactants as well as their relative distribution within epithelial basement membranes.69,70
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree